Covalent bonding - CushmanChemistry
advertisement

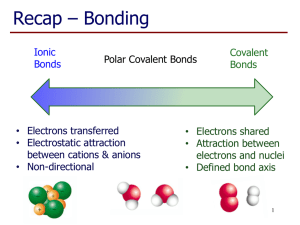
Covalent bonding By Cush Molecular/Ionic compounds Molecular compounds have very low boiling and melting points. Ionic compounds however have very high boiling and melting points. Molecular formula Molecular formulas tell you what the element is and how many atoms are present in a molecule. Ex: Water – H2O Electron sharing in covalent bonds This occurs when two or more elements share electrons. The result is equally matched atoms leaving you with a compound such as H2. Dot Structure The dots represent the number of electrons a element has to give away for compounding. Double and triple covalent bonds Double covalent bonds are formed by the sharing of two pairs of electrons between two atoms. Triple covalent bonds are formed by the sharing of three pairs of electrons between two atoms. Dissociation Energy Dissociation energy is related to the strength of a covalent bond because of its constituent atoms. Oxygen Atoms The ozone is three oxygen atoms bonded together. The atoms bond because there is three of them in the ozone. Exceptions to the Octet rule One exception is to be able to predict all formulas and to be able to account for all molecular structures involving covalent bonds. VSEPR Theory The VESPR Theory helps us predict the shape of individual molecules based up the extent of electron –pair electrostatic repulsion. Sources cited http://www.utc.edu/Faculty/GretchenPotts/chemistryhelp/compounds.htm http://science.jrank.org/pages/4403/Molecular-Formula.html http://www.visionlearning.com/library/module_viewer.php?mid=55 http://www.ehow.com/how-does_4568765_electron-dot-diagramwork_.html http://www.tutorvista.com/content/chemistry/chemistry-i/chemicalbonding/double-covalent-bond.php http://www.tutorvista.com/content/chemistry/chemistry-i/chemicalbonding/triple-covalent-bond.php http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/react2.m http://instaar.colorado.edu/outreach/ozone-oceans/ozone.html http://chemed.chem.wisc.edu/chempaths/GenChem-Textbook/Exceptions-to-the-OctetRule-573.html http://en.wikipedia.org/wiki/VSEPR_theory