Task 3- Chemistry
advertisement

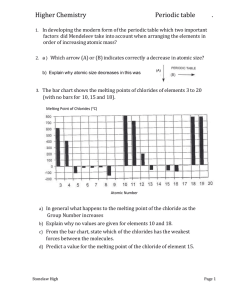
Task 3- Trends in the Periodic Table 1. Complete the table 1 below Element Proton Number Z Li Na K Rb Cs First Ionisation Energy (kJmol-1) 520 496 419 403 376 3 11 19 37 55 2. Plot a graph of first ionisation energy against the proton number, Z. (2 marks) First Ionisation Energy 600 500 400 300 First Ionisation Energy 200 100 0 3 11 19 37 55 Figure 1: First Ionisation Energy based on the Proton Number 3. With reference to the graph, as you move down the group does the ionisation increase or decrease? Include a reason for your answer. (4 marks) As shown in the graph, as you move down the group the First Ionisation Energy decreases. The First Ionisation Energy for Lithium is 520kJmol-1 a relatively high number in comparison to the other elements. Sodium is the next element as you go down group 1. The First Ionisation Energy of Sodium is 496kJmol-1. This is a difference of 24 kJmol-1, showing a decrease in the First Ionisation Energy. This trend is continued as you go further down group 1 in the periodic table. Potassium is the next element down group 1 and it has a First Ionisation Energy of 419 kJmol-1,.a decrease of 77 kJmol-1. The decrease in the First Ionisation Energy down a group can be explained in terms of the distance from the nucleus to the electron being increased. The further from the nucleus the outermost electron is, the less attraction there is between the electrons and the nucleus, therefore, the less energy is required to remove the electron. The electron shells between the valence shell and the nucleus also contribute a ‘shielding’ effect. This reduces the attraction between the valence electrons and the attraction force of the nucleus. Resulting in less Ionisation Energy being needed to remove the valence electron. 4. Using the internet, find values for the atomic radius of the above elements. Plot a graph of proton number against atomic radius. You may plot it on the first graph. (4 marks) Element Li Na K Rb Cs Proton Number 3 11 19 37 55 Atomic Radius (pm) 145 186 220 248 260 Atomic Radius 300 250 200 150 Atomic Radius 100 50 0 3 11 19 37 55 Figure 2: The Atomic Radius in comparison to the Proton Number 5. Does the atomic radius increase or decrease down the group? (1 mark) As shown in Figure 2, the atomic radius increases as you go down the group. 6. Using the above information, describe the reactivity of the elements down the group, giving examples. You will need to also provide a chemical equation and describe the observations (6 marks) The reactivity of the elements increases as you go down a group on the periodic table. Electrons are held in their shells by an attractive force from the protons in the nucleus. Whenever metals react, they do so by losing their valence electron(s). The valence electron in lithium (the first element in group 1) is relatively close to the nucleus and the force holding the electron in place is quite strong. Therefore, the valence electron is relatively hard to remove which means that lithium is not as reactive as those further down group 1, for example, Potassium. Potassium has 4 electron shells and 1 valence electron. Even though it has the same amount of valence electrons and lithium, it is more reactive because there is less force attracting the valence electron to the nucleus due to the ‘shielding’ effect from the electron shells. 7. Would group 2 show a similar trend to group 1? Find evidence on the internet to support your answer. (4 marks) Group 2 would show a similar trend to group 1 as you are still going down a group on the periodic table that is part of the ‘metals’ section of the periodic table. 8. Complete Table 2 Below (1 mark) Element Proton Number Z Li Be B C N O F Ne 3 4 5 6 7 8 9 10 First Ionisation Energy (kJmol-1) 520 900 801 1806 1402 1314 1681 2081 9. Plot a graph of First Ionisation Energy against the proton number, Z. (2 marks) First Ionisation Energy 2500 2000 1500 First Ionisation Energy 1000 500 0 3 4 5 6 7 8 9 10 Figure 3: First Ionisation Energy against the Proton Number 10. With reference to the graph, as you move across the period (left to right) does the ionisation increase or decrease? Include a reason for your answer. (4 marks) As you move across the periods the ionisation energy generally increases. Moving from one element to the next across a period, an additional proton is added to the nucleus, which increases the core charge. Therefore, the electrons in the outermost shell will have a stronger attraction to the nucleus. As a result of this, it will require more energy to remove an electron from the atoms with the larger atomic number in a particular period. However, there are a few exceptions. This includes Boron, which has an unusually low ionisation energy and Carbon which has an unusually high ionisation energy. 11. Complete table 3 (1 mark) Element Proton Number Z Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Electronegativity (Pauling) 0.98 1.57 2.04 2.55 3.04 3.44 3.98 0 12. Plot a graph of the electronegativity against the proton number, Z. (2 Marks) Electronegativity (pauling) 4.5 4 3.5 3 2.5 Electronegativity (pauling) 2 1.5 1 0.5 0 3 4 5 6 7 8 9 10 13. With reference to the graph, as you move across the period (left to right) does the electronegativity increase or decrease? Give a reason for your answer (4 marks) As you move across the period the electronegativity increases. The electronegativity increase as a proton is being added to the nucleus creating a stronger attraction. As the attraction force of the nucleus increases, the electrons have a stronger attraction to the nucleus. Resulting in a larger electronegativity. 14. How would you describe the reactivity across the period? Give a reason for your answer. (3 marks) The reactivity decreases as you go across the period. This is because the ionisation energy increases. The higher the ionisation energy, the harder it is to remove the valence electrons. For an element to react, it has to lose its valence electrons. If they are harder to remove, it will have a lower reactivity. 15. Explain why Neon does not follow the trend? (1 mark) Neon does not follow the trend because it has a full outermost shell. Because it has a full outermost shell, it doesn’t need to attract electrons to it to fill the outermost shell.