Document
advertisement

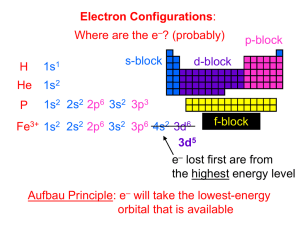
In hydrogen all orbitals in the same shell have the same energy: s 3 2 1 p d but once some orbitals contain electrons the energies of other orbitals are affected due to repulsion so the p and d orbitals have higher energy than the s orbitals of the same shell: s 3 p d 2 You need to copy this diagram 1 Hydrogen 1s1 Helium 1s2 Lithium 1s2 2s1 Beryllium 1s2 2s2 Boron 1s2 2s2 2p1 Carbon 1s2 2s2 2p2 Nitrogen 1s2 2s2 2p3 Oxygen 1s2 2s2 2p4 Try to write down the electronic configurations of : Neon (z = 10) 1s2 2s2 2p6 Sodium (z = 11) 1s2 2s2 2p6 3s1 Silicon (z = 14) 1s2 2s2 2p6 3s2 3p 2 Potassium (z = 19) 1s2 2s2 2p6 3s2 3p 6 4s 1 You may have expected the final electron in potassium to be in the 3d sub-shell 1s2 2s2 2p6 3s2 3p 6 3d 1 but potassium is so similar to sodium that the electronic structure should also be similar with a outer s electron Na K 2 1s 2 2s 6 2p 1 3s 1s2 2s2 2p6 3s2 3p 6 4s 1 The explanation is that the increase in energy of the 3d sub-shell puts it higher than the 4s. 4f d 4 3 2 p s 1 NB 3d sub-shell higher than the 4s. Potassium d 4 3 2 p s 1 1s2 2s2 2p6 3s2 3p6 4s1 2 8 8 1 This accounts for the odd series of lengths of periods in the Periodic Table : 2, 8, 8, 18 when the maximum number of electrons per shell is 2, 8, 18, 36 Bromine for example is the 17th element across the PT but has only 7 outer electrons. Bromine d 4 3 2 p s 1 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p5 2 8 18 7