Environmental and genotypic effects in Solidago altissima on
advertisement

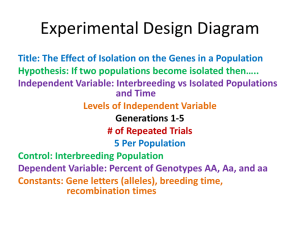
Effect of genotype and environment on the abundance of a specialist aphid in Solidago altissima Brian Bonville and Ray S. Williams Appalachian State University Introduction Community Genetics Certain species of plants are the foundation of a given ecosystem Their genetic variation may govern population responses in animals that depend on them Red Goldenrod Aphid (Uroleucon nigrotuberculatum) • Insect communities are known to be affected by intraspecific genetic variation in Solidago altissima (Crutsinger et al, 2006). • The abundance of herbivores such as aphids can vary between genotypes (Genung et al. 2012, Williams and Avakian 2015). • Though insect responses are observed, the mechanisms behind insect choice of genotypes is relatively unknown. • In addition, the role of environment and its interaction with genotype in shaping insect responses is not well understood. Terpenes • Allelochemicals acting as defensive agents, signaling chemicals, or attractants to insects. • Previous research with S. altissima found terpenes positively correlated with aphid abundance in S. altissima (Williams and Avakian 2015). Objectives • Investigate the effects of genotype (G) and environment (E) and GXE on Uroleucon nigrotuberculatum abundance on S. altissima by the addition of nitrogen and phosphorous to soil. • To examine the effects of G, E, and GXE on terpenes. Methods • In May 2014, six genotypes of tall goldenrod were planted in a common garden design. • Plants were administered either nitrogen, phosphorous or no nutrients (control). There were three replicates of each treatment. Plants were spaced 0.33m apart. • The total of 54 plants were broken into three blocks. • Aphids colonized the plants naturally. Block 3 Block 2 Block 1 54 2 N 53 5 C 52 4 N 51 4 C 50 2 P 49 4 P 48 2 C 47 1 N 46 6 N 37 1 P 38 3 N 39 6 C 40 1 C 41 5 N 42 3 C 43 6 P 44 3 P 45 5 P 36 5 C 35 6 N 34 3 N 33 3 P 32 4 N 31 2 P 30 4 C 29 2 C 28 1 N 19 4 P 20 1 P 21 6 P 22 5 N 23 1 C 24 3 C 25 2 N 26 5 P 27 6 C 18 6 C 17 5 N 16 4 N 15 3 P 14 1 P 13 6 P 12 3 C 11 2 P 10 1 N 1 2 N 2 4 C 3 5 P 4 5 C 5 2 C 6 3 N 7 6 N 8 4 P 9 1 C Field Data Collection • Aphid abundance was monitored every three days throughout the season. • Biomass estimates and leaf samples were taken during aphid abundance. • Plants were harvested and biomass determined at the end of season. Photo- Ricochet Science Productions Chemical Analyses • Leaves were ground in pentane for GC analysis in a Schimadzu GC-14A gas chromatograph. • Compounds were identified using analytical standards and quantified with an internal standard. Statistical Analyses • Two-way ANOVA with repeated measures (SAS 9.4) was used to test for effects of genotype (G), nutrient treatment (E), and G XE interaction on aphid abundance, terpene concentration and plant biomass. Results where 0.05 < P < 0.1 reported as marginally significant. Results Aphid Abundance during season 3000 Total aphids 2500 2000 1500 1000 500 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 Day Aphid Abundance Jul. 9 Aphid Abundance Sept. 3 Fertilization P= 0.1900 Fertilization P= 0.044 Genotype P=0.2418 Genotype P=0.4352 Fertilization*Genotype P=0.0272 Fertilization*Genotype P=0.0989 Meanaphid aphid abundance by treatment Mean abundance by treatment July 9 250 Mean aphid abundance 200 150 Nitrogen Control Phosphorus 100 50 0 2.000 2 4.000 4 5.000 5 Genotype 3.000 3 6.000 6 Biomass estimate Jul. 9 Biomass estimate Sept. 3 Fertilization P= 0.0009 Fertilization P<0.0001 Genotype P<0.0001 Genotype P<0.0001 Fertilization*Genotype P=0.7721 Fertilization*Genotype P=0.8698 Biomass September 3 α-pinene July 9 α-pinene Sept 3 Fertilization P= 0.0816 Fertilization P= 0.0204 Genotype P=0.0008 Genotype P<0.0001 Fertilization*Genotype P=0.0196 Fertilization*Genotype P=0.6331 β-pinene β-pinene Fertilization P= 0.2187 Fertilization P= 0.0249 Genotype P=0.0006 Genotype P<0.0001 Fertilization*Genotype P=0.1311 Fertilization*Genotype P=0.6647 Germacrene D Germacrene D Fertilization P= 0.1251 Fertilization P= 0.0707 Genotype P=0.0004 Genotype P=0.0080 Fertilization*Genotype P=0.4198 Fertilization*Genotype P=0.9600 P-cymene P-cymene Fertilization P= 0.4023 Fertilization P= 0.0196 Genotype P=0.1212 Genotype P<0.0001 Fertilization*Genotype P=0.7188 Fertilization*Genotype P=0.5956 All four terpenes All four terpenes Fertilization P= 0.1317 Fertilization P= 0.0289 Genotype P=0.0012 Genotype P=0.0041 Fertilization*Genotype P=0.3892 Fertilization*Genotype P=0.9543 Summary • At the first sampling date fertilization did not significantly influence aphid abundance. • However, there was a significant GXE effect in July on aphid abundance. In addition, a marginal GXE effect was seen in September. • As the season progressed, more aphids were found on plants with N addition, regardless of genotype. The N fertilized plants were also significantly larger. • A genotype effect was seen in terpene concentrations both in July and September and a fertilization effect arose In July. • A higher mean terpene concentration was found in phosphate fertilized plants. Discussion • Overall aphid population was affected by nutrient treatment and a GXE effect was demonstrated the early growing season. • For terpene concentration the effect of genotype was most significant, and fertilization was seen to be significant later in the season. • Phosphate fertilized plants had significantly higher mean terpene concentration. Flowering may deplete plants of phosphorus. Phosphorus may be integral in terpene production. Future Work • Awaiting analyses of C:N and N content in plants to shed more light on aphid plant colonization. • Run Partial Least Squares Regression for individual terpenes, nutrients and aphid abundance. A multivariate correlative analysis. • Will do linear regression analysis between aphids and nutrients, individual terpenes, and biomass. • GC analysis on terpene content in aphids. Acknowledgements Williams Lab Ray S. Williams Julie Ragsdale Marae Lindquist Jacob Pawlik Bryan Taylor Other Mike Madritch Howie Neufeld Jerry Meyer Quinn Griffin Delaney Trimble Funding: Appalachian State Office of Student Research