AREA OF STUDY 2 chapter 10 summary notes
advertisement

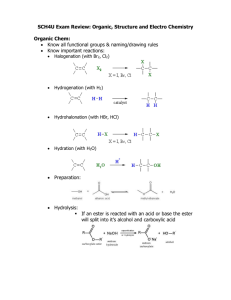
VCE Chemistry Unit 3, Area of Study 2 2013 Chapter 10 Summary Notes (part 1) Organic reactions: pathways to new products Key Knowledge addressed in this Chapter: Common reactions of organic compounds: addition reactions of alkenes, substitution reactions of alkanes and primary chloroalkanes, oxidation of primary alkanols, esterification; Principles of fractional distillation; Organic reaction pathways including the production of esters from alkenes, condensation and polymerisation reactions that produce large biomolecules; Reactions of Alkanes (substitution reactions) All chemical reactions require the__ _______________ of existing bonds- that is, overcoming the electrostatic attraction holding atoms or ions together. The _______________ this force of attraction, the _____________ energy required. This requirement of energy explains why even the combustion of the most flammable alkanes needs to be started with a spark. Because alkanes are ________________ and contain no shared electron pairs, they are not susceptible to attack by electron-deficient or electron-rich reagents. Thus alkanes are _______________ to attack by most oxidants and reductants, as well as by most acids and bases. However, they do react with oxygen and with halogens such as Chlorine and Bromine. The formation of Chloroalkanes When alkanes react with chlorine, the chlorine atoms cannot simple add on to the molecule because alkanes are saturated. They therefore must _____________ a hydrogen atom. Hence these are classified as ________________ ____________________. Because of their strong ____________ bonds, alkanes will only undergo substitution reactions at reasonably high temperatures and/or in the presence of ___________, particularly UV-light as it is high energy radiation. So, if a mixture of methane and chlorine is kept at room temperature in the dark, no reaction will occur. VCE Chemistry Unit 3, Area of Study 2 2013 The substitution reaction between Chlorine and Methane: As the number of carbons gets bigger, there will be more possible structural isomers that can form, so increasingly more complex mixtures of products will be formed. It is important to remember that in these reactions, however, the total number of _____________ atoms in each molecule is unchanged, because the Cl atoms only replace H atoms. The reaction between Chlorine and Propane: The formation of amines Once a chloroalkane is prepared, we have a _____________ molecule that is more reactive than its parent alkane. This can be reacted with __________________ (_________) to form an amine. The best conditions for this reaction involve dissolving the chloroalkane in ethanol and reacting this with excess ammonia in a sealed tube at 100oC. The amino group then replaces the chloro group. VCE Chemistry Unit 3, Area of Study 2 2013 The placement of the amino group depends on the placement of the chlorine group because this is a ________________________ _________________. The formation of alcohols from chloroalkanes One method of producing an alcohol is to react the corresponding chloroalkane with water. In this reaction, a hydroxyl group is directly substituted for the chloro group. This is not only classified as a substitution reaction but also a _____________________ reaction because water is a reactant. Hydrolysis of hydrocarbons by water is a very slow reaction even when the temperature of the reaction is raised. The reaction of 1-chlorobutane is: As you can see, HCl (H+ & Cl-) is produced. Usually, this reaction is carried out in the presence of aqueous sodium hydroxide, so the reaction is faster. The reaction then is: As you can see, Cl- is produced. The formation of chloroalkanes from alcohols Interestingly you can also produce a chloroalkane from its corresponding alcohol. Although dry HCL gas will work, the best reagent is probably phosphorous pentachloride PCl5 VCE Chemistry Unit 3, Area of Study 2 2013 The production of carboxylic acid As discussed earlier, winemakers go to great lengths to prevent oxygen from entering their wines, as it ruins the wine by turning it into vinegar. This is because ethanol can be oxidised by oxygen to ethanoic acid (vinegar). Other strong _____________ like acidified potassium dichromate solution or acidified potassium permanganate solution also cause this reaction to occur. If you were aiming to prepare propanoic acid, which has three carbon atoms, for example, you would need to start with propan-1-ol, as the carboxylic functional group needs to be at the end of the hydrocarbon chain and during these oxidation reactions the total number of carbon atoms does not change. (You substitute the Oxygen atoms for the Hydrogen atoms only). Reactions of Alkenes (addition reactions) The presence of the ____________ bond in an unsaturated alkene molecule means that they can undergo reactions in which atoms or groups of atoms are added across the bond to form a saturated compound. These are ____________ ________. The hydrogenation of Ethene Since hydrogen is the substance that adds on to the ethene molecule, this is also a _____________ reaction. However, this is not a particularly useful reaction as you can make far more products from ethene than ethane! VCE Chemistry Unit 3, Area of Study 2 2013 Catalysts Substances cannot react unless their particles are in direct physical contact. Hence, all reactions require collisions between the reactant particles. A __________ is a substance that is added to speed up a reaction but which is not consumed in the process. We will study catalysts in much more detail in unit 4, however it’s important to know that most reactions organic chemicals undergo require a catalyst and to understand why this is the case. An example to help you understand: One important industrial use is in the hydrogenation of vegetable oils to make margarine, which also involves reacting a carbon-carbon double bond in the vegetable oil with hydrogen in the presence of a nickel catalyst. Ethene molecules are adsorbed on the surface of the nickel. The double bond between the carbon atoms breaks and the electrons are used to bond it to the nickel surface. Hydrogen molecules are also adsorbed on to the surface of the nickel. When this happens, the hydrogen molecules are broken into atoms. These can move around on the surface of the nickel. If a hydrogen atom diffuses close to one of the bonded carbons, the bond between the carbon and the nickel is replaced by one between the carbon and hydrogen. That end of the original ethene now breaks free of the surface, and eventually the same thing will happen at the other end. VCE Chemistry Unit 3, Area of Study 2 2013 As before, one of the hydrogen atoms forms a bond with the carbon, and that end also breaks free. There is now space on the surface of the nickel for new reactant molecules to go through the whole process again. The addition reactions of ethene Ethene can be a starting point for the production of chloroethane and ethanol, as well as ethanoic acid and ethanamine. VCE Chemistry Unit 3, Area of Study 2 2013 The reaction pathways are similar for other alkenes, but remember the total number of Carbon atoms doesn’t change in these reactions. For example but-1-ene: The bromine test Like chlorine, bromine can add across the carbon-carbon double (or triple) bond of an alkene (or alkyne). Bromine is a brownish red colour, but the product is colourless. Therefore, if the colour of bromine disappears when it is mixed with an unknown hydrocarbon, we can conclude that the hydrocarbon is unsaturated. VCE Chemistry Unit 3, Area of Study 2 2013 Chapter 10 Summary Notes (part 1) Organic reactions: pathways to new products Esters The ester family is the family of molecules that contain the ___________ ____________ __________, which may be written as _____________ or __________ in the semistructural formula of an ester. Esters can be synthesised quite easily, and have a variety of uses, including as artificial flavours in products such as ice-cream and confectionary. Some are used to give products enticing aromas. The ester in your box above is found in pineapples, peaches and apricots. The ester group formed is also termed an ____________ ________, as it links (joins) an alcohol molecule and a carboxylic acid molecule, from which an ester is made. Synthesising esters An ester can be produced from an ___________ and a ________________ _______ by heating the mixture in the presence of an acid catalyst. The catalyst is usually dry hydrogen chlorine gas or concentrated sulphuric acid. Note:_____________________________________________________________________________ _________________________________________________________________________________. The ester produced above is called _____________ ___________. This ester smells like bananas. VCE Chemistry Unit 3, Area of Study 2 2013 The balanced equation for this reaction is: In the interaction between the two different functional groups, a water molecule is released. Reactions in which two molecules join as a result of a reaction between their functional groups, which results in the release of a small molecule, are _____________ reactions. When a condensation reaction also produces an ester, it is also classified as an ______________ reaction. The general equation for the formation of an ester is: Naming esters 1. First we give the name of the alkyl group that comes from the alcohol used to prepare the ester, R’OH. 2. Second we give the name of the carboxylic acid from which the ester has been produced, RCOOH, except we change the end of the name the acid from ‘oic acid’ to ‘________’. For example: VCE Chemistry Unit 3, Area of Study 2 2013 The hydrolysis of an ester Just as an ester can be built up from a carboxylic acid and an alcohol, under the right conditions it can be _____________ _________ back into the carboxylic acid and alcohol from which it was produced. The breakdown of an ester requires a water molecule to be inserted back into the ester functional group from which it is lost. This reaction also requires a suitable catalyst, which can be an acid or a base. In the case of a base, the salt of the carboxylic acid is produced instead of the acid itself. The general overall equations for these hydrolysis reactions are: For example: Acid catalysed hydrolysis of butyl methanoate Base-catalysed hydrolysis of butyl methanoate VCE Chemistry Unit 3, Area of Study 2 2013 Aspirin –an ester Early civilisations knew that drinking a tea made from the bark of a willow tree relieves fever and inflammation. In the 19th century, the compound _________ was extracted from willow bark. In the body, salicilin converted to __________ _________. Unfortunately this acid was bitter and caused stomach bleeding. Eventually it was found that by __________ salicylic acid, the side effects were considerably reduced. Acetylsalicylic acid, or aspirin as it is commonly known, is the largest selling pain relief (analgesic) in the world. The synthesis of aspirin