Goal 5: Organic Compounds

ORGANIC
COMPOUNDS
Organic chemistry is the chemistry of carbon.
• Carbon always forms 4 covalent bonds.
• It can produce long chains that form the backbone of the important molecules in a cell.
• Examples: sugars, fats, proteins, nucleic acids
THE STRUCTURE OF A MOLECULE IS
RELATED TO ITS FUNCTION
1. Functional groups- a recognizable group of atoms attached to the carbon skeleton.
They may make the molecule polar or change its pH.
Examples: a. Hydroxyl (OH) —found on alcohols b. Carbonyl (C=O or C=OH) –found on the end of the molecule called aldehyde, when found in the middle called ketone c. Carboxyl (C=OOH) —found on sugars, fats and proteins d. Amine (NH
3
) —found on proteins e. Phosphate (PO
4
) —found on nucleotides, ATP f. Methyl (CH
3
) —found on fats
2. Monomers form polymers
• Monomer-mono = one, these are small subunits used to build larger molecules these subunits are stored in cells until needed
• Polymer-poly = many, these are huge molecules, built by joining subunits, used for either structural or functional parts of cells
TYPES OF REACTIONS:
1. electron transfer-one or more electrons are taken from one molecule and given to another oxidation-occurs on the molecule that gives away electrons reduction-occurs on the molecule that takes electrons
2. condensation (dehydration)-joining two molecules by removing a water molecule
3. cleavage (hydrolysis)-breaking apart a molecule by inserting a water molecule
All reactions either require energy or give off energy as they progress.
1.
Exergonic-give off energy as they happen, occur spontaneously these are catabolic reactions-they release energy by breaking down molecules
2. Endergonic-require energy to proceed, not spontaneous these are anabolic reactions-simple molecules combine to form complex ones.
* the rate of any reaction is determined by temperature, concentration, catalysts
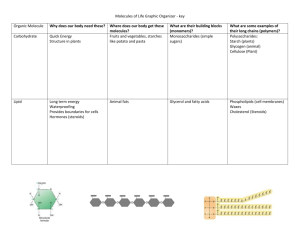
THE 4 MAJOR MOLECULES OF LIFE
1. Carbohydrates-
• starches and sugars
• used by cells as structural material, transport or to store energy
• made of C, H, O in a ratio of 1:2:1
Monosaccharides-
• mono means one, saccharide means sugar.
• examples-glucose, fructose, ribose
C
6
H
12
O
6
C
6
H
12
O
6
C
5
H
10
O
5
Disaccharides- 2 sugars bonded together examples:
• Sucrose = fructose + glucose
• Lactose = glucose + galactose
• Maltose = glucose + glucose
Polysaccharide-
• Means many sugars, these are complex and very large examples:
• In plants-
• starch -consists of long chains of glucose stored in plastids.
Represents stored energy.
A sugar can be broken off the chain when needed by hydrolysis.
Starches are helical (called alpha configuration)
• Cellulose -commonly known as fiber, it is part of the cell walls of plant, most abundant organic compound on earth. Few organisms have the necessary enzymes that can break it apart.
It is made by joining sugar in straight chains called the beta configuration.
• In Animals
• glycogen -excess sugar is stored in liver and muscle cells.
This molecule has long branching chains. In humans glycogen is depleted daily and must be replaced by eating
• chitin -used by arthropods to build exoskeletons.
Also found in cell walls of fungi.
2. Lipids-
• store huge amounts of energy (one gram stores more than twice starch),
• made of glycerol and fatty acids.
• animals store fat in adipose cells
• fat also cushions organs and insulates the body
a. Fatty acids-long carbon chain with a carboxyl functional group (-COOH) b. Triglyceride-one complete fat molecule, made of 3 fatty acids chains c. Unsaturated fat-has one or more double bonds on its chain, usually a liquid, comes from plants and fish d. Saturated-no double bonds along the chain, usually a solid, comes from animals
e. Phospholipids-made with 2 fatty acids and a phosphate linked to a glycerol.
f. Sterols-contain 4 carbon rings, vary in their functional groups.
•
Examples include hormones and cholesterol
(component of cell membranes)
g. Waxes-long fatty acids chains linked to alcohols
• coating that protects and lubricates
• plant’s cuticle-helps to conserve water
• beeswax to make honeycomb
• bird feathers kept dry
3. PROTEINS
Used for structural support, transport, communication, movement, defense, enzymes
• A single human cell has tens of thousands of proteins
• Made by joining amino acids- 20 are common. Some are made by cells, others must be eaten.
Peptide Bond-a covalent bond formed when two amino acids are linked together
Polypeptide-long chains of amino acids.
• A complete protein is one or more polypeptides folded and coiled into place
Primary structure-the amino acid chain. The order they go in is determined by DNA.
• The order determines function of the protein
• example: substituting one amino acid in hemoglobin causes sickle cell anemia
Secondary structure-coils and pleats, shape is held together by hydrogen bonds.
Tertiary structure-folded on top of itself
Quaternary structure-2 or more of these polypeptides bonded together.
• Example: hemoglobin is 4
• protein shape determines its function
Variations in final proteins:
• lipoproteins-protein combined with a fat
• glycoproteins-protein attached to sugars
Denaturation-when a protein unravels due to environmental conditions
• pH, temperature
• Loss of shape means loss of function
ENZYMES
Definition: catalytic proteins, they cause reactions to proceed faster.
All reactions require the breaking and making of bonds. Energy is required to do this and enzymes reduce the energy needed.
They change the rate of the reaction but not the product.
EX: SUCROSE + WATER SUCRASE > GLUCOSE + FRUCTOSE
Characteristics of enzymes:
• work on the normal reaction (not making a new one)
• can be used over and over
• the same enzyme usually works for forward and reverse reaction
• selective about substrates
Structure-one or more active sites. This is the catalytic center
(reactive place)
Enzymes function best when cell’s environment stays within a limited range
• temperature-each has an optimal range, when it is exceeded denaturation occurs
• pH-optimal pH is 6-8 (except gastric enzymes)
• Inhibitors-slow enzymes by controlling their active site
• competitive-chemical mimics, they resemble the substrate and compete for the active site
• example: penicillin blocks the active site of the enzyme needed to make the cell wall of the bacteria cell
• Noncompetitive-react by changing the shape of the active site
• Allosteric (noncompetitive)-binds to enzyme at another site but still changes the active site so it can’t function
THINGS THAT HELP ENZYMES WORK
BETTER:
Coenzymes-organic molecules known as vitamins
Cofactors-inorganic molecules- known as minerals
4. NUCLEIC ACIDS
Known as “information” molecules, DNA and RNA are examples.
They are built from monomers called nucleotides which consist of a sugar, a phosphate and a base
4 bases are used in DNA:
• Adenine
• Guanine
• Thymine
• Cytosine
*RNA uses uracil instead of thymine
G and A are double ringed and called purines
T and C are single rings and are called pyrimidines.
DIFFERENCES IN DNA AND RNA:
DNA-double stranded, helical, made with sugar called deoxyribose
RNA-single stranded, helical, made with sugar called ribose