1 - Learning
advertisement

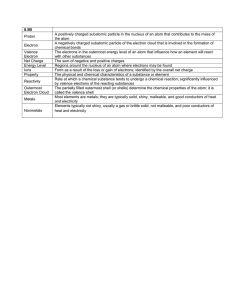
GRADE 10 SCIENCE THE PERIODIC TABLE The Periodic Table is to arranged in a way that enables chemists to understand patterns in the properties of elements. The elements are laid out in order of Atomic (proton) Number. Many of the similarities and differences in the properties of elements can be explained by the electronic structure of the atoms - electron configuration The main structural features of the periodic table are: Columns of similar elements called Groups. They are usually similar chemically and physically BUT there are often important trends in physical properties and chemical reactivity up/down a group. Complete horizontal rows are called Periods. Usually consist of a range of elements of different character. There are important trends from left to right across a period e.g. the most important overall change is from metallic non-metallic element character. The metal/non-metal divide – Know it! Elements on the left are METALS Elements on the right are NON-METALS Some elements along the divide are referred to as SEMI-METALS or METALLOIDS. They have mixed metal/non-metal character. PERIODICITY Many physical and chemical properties of the elements follow a sequence and change systematically as one moves across from one side of a period to the other. This is called periodicity. We study a few of these properties through periods 2 and 3 Density 3,5 TRENDS: - General increase in density from Group 1 to the middle of the periodic table 3 Density (g.cm-3) 2,5 - General decrease from the middle of the table to Group 8 2 1,5 - Density in a group in Period 3 elements is higher than in Period 2 1 0,5 Element at top of group 0 0 1 2 3 4 5 6 7 9 Elements 8 Group Number at bottom of group Melting Points 4000 TRENDS: 3500 - Melting points of metals increase across a period Melting Point (°C) 3000 2500 - Melting points of non-metals decrease across a period 2000 - Melting points of metals decrease down groups 1500 1000 - Melting points of non-metals increase down groups 500 0 -500 0 1 2 3 4 5 Group Number 6 7 8 9 Boiling Points TRENDS: 6000 - Boiling points of metals increase across a period Boiling Point (°C) 5000 - Boiling points of non-metals decrease across a period 4000 3000 - Boiling points of metals decrease down groups 2000 - Boiling points of non-metals increase down groups 1000 0 -1000 0 1 2 3 4 5 6 7 8 ie SAME as MELTING POINT 9 Group Number Atomic Radius Definition: Distance between the nucleus and the outermost orbital of an atom True atomic radius Group 8 exist as free atoms Covalent radius Diatomic molecules ½d= radius Metallic radius Atoms in metallic lattice TRENDS: - increase down a group (extra shell added in each period) - decrease across a period (adding electrons to the same shell but, increased negative charge means increased electrostatic attraction and thus ‘contracts’ the atom Formulae of Halides The halogens (Group 7 – F2, Cl2, Br2, I2) are reactive and form halides (fluoride, chloride etc) when reacting with other elements (except with unreactive Group 8). Chlorides are used as the example below. Group 1 2 3 4 5 6 7 Period 2 Halide LiCl BeCl2 BCl3 CCl4 NCl3 OCl2 FCl Period 3 Halide NaCl MgCl2 AlCl3 SiCl4 PCl3 SCl2 Cl2 Ratio element:Cl 1 2 3 4 3 2 1 NOTE: Peak of combining power occurs at changeover from metals to non-metals Formulae of Oxides The same trend as for the halides is noted – this is true of many of the compound form – combining power increases across the metals and decreases across the non-metals Group 1 2 3 4 5 6 7 Period 2 Oxide Li2O BeO B2O3 CO2 varies O2 F 2O Period 3 Oxide Na2O MgO Al2O3 SiO2 P4O6 varies Cl2O Ratio element:O 0.5 1 1.5 2 1.5 - 0.5 Ionisation Energy Definition: The ionization energy of an element is the amount of energy required to remove an electron from a gaseous atom to form a cation. The higher the ionisation energy, the more difficult it is to remove an electron from an atom It is possible to remove successive electrons from an atom: First eFirst ionisation energy M M+ + eSecond eSecond ionisation energy M+ M2+ + eThird eThird ionisation energy M2+ M3+ + eWe only look at the trends in first ionisation energy: TRENDS: - ionisation energies increase across each period – as an extra proton and electron are added to an atom, electrostatic attraction increases. - large drop at the start of each new period. Electrons are being added to a new shell which is further from the nucleus thus experience weaker electrostatic forces Electron affinity Definition: The energy change when an electron is added to a gaseous atom to form an anion. (ie a measure of the ability of an atom to accept an electron) TRENDS: - electron affinity increases across each period – those of metals are generally lower than those of non metals - electron affinities of the halogens are highest – by accepting an electron, they attain the noble gas configuration of the noble gas next to them Electronegativity Definition: The ability of an atom in a compound to attract the bonding electron pair(s) TRENDS: - increases across each period halogens are highest – decreases down a group CHEMICAL PROPERTIES among ELEMENTS Reactivity determined by the no of valence electrons that need to be lost or gained to achieve a full outer shell. - lose electrons - no of protons > no of electrons - positive ion (CATION) formed - gain electrons - no of electrons > no of protons - negative ion (ANION) formed Elements in same group – same no of valence electrons, so they will lose/gain the same no of electrons to achieve a full outer shell, so reactivity is similar. GROUP 1 – alkali metals - all have 1e in outer shell. - lose 1 electron when they react – form cations with a 1+ charge - react vigorously with water to give hydrogen gas and an alkaline solution of the metal hydroxide: eg. 2Li + 2H2O 2LiOH+ H2 - burn strongly in oxygen to form solid oxides: eg. 4Li + O2 2Li2O - ionisation energy is low in Group 1, so losing an electron to form +1 ion is easy, so elements in Group 1 are very reactive. - ionisation energy decreases as we go down the group (shell size increases), so reactivity INCREASES down the group. - physical properties – soft, low density (float on water), silvery colour, low melting point GROUP 2 – alkali earth metals - all have 2e in outer shell. - lose 2 electrons when they react – form cations with a 2+ charge - less reactive than Group 1 since more energy needed to remove second electron. - reactivity with water varies. Be – not reactive Mg – very slow with cold water, vigorous with steam Mg + 2H2O(l) Mg(OH)2+ H2 Mg + H2O(g) MgO + H2 Ca, Sr, Ba react more vigorously with cold water Ca + 2H2O Mg(OH)2+ H2 Group 2 hydroxides are only very slightly soluble. - burn in oxygen to form solid oxides: eg. 2Mg + O2 2MgO - ionisation energy decreases as we go down the group (shell size increases), so reactivity INCREASES down the group. - physical properties – silvery colour but harder, more dense, higher melting than group 1 GROUP 7 – halogens - form diatomic molecules (F2, Cl2, Br2, I2) – poisonous! - all have 7e in outer shell. - gain 1 electron when they react – form anions with a 1- charge - react with Group 1 metals to form salts. 2Li + Cl2 2LiCl - Since an electron is being, reactivity depends on electron affinity. - Electron affinity decreases down the group, so reactivity DECREASES down the group. Fluorine is frequently explosive – we don’t use it. - Physical properties: - low melting and boiling points (increase as you go down group) At room temp, F2, Cl2 - gases, Br2 - liquid, I2 - solid, - colour varies F2 – colourless, Cl2 - green-yellow, Br2 - red-brown, I2 – purple-black GROUP 8 – noble/inert gases - have a full outer shell – no gain or loss of electrons, unreactive or INERT - Physical properties – low boiling point, colourless, gases at room temperature