Chapter 3 Science Vocabulary: Definitions & Examples
advertisement

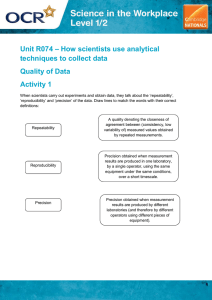
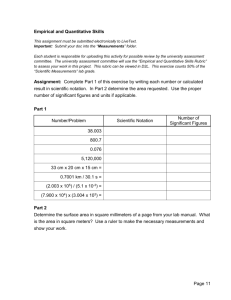
Chapter 3 Vocabulary •Measurement •Scientific notation Measurement a quantitative description that includes both a number and a unit Examples: Length (4 meters), age (15 years), or daily temperature (31°C) Scientific Notation an expression of numbers n in the form m x 10 , where m is equal to or greater than 1 and less than 10, and n is an integer Chapter 3 Vocabulary • Accuracy • Precision • Accepted Value •Experimental Value Accuracy the closeness of a measurement to the true value of what is being measured Precision describes the closeness, or reproducibility, of a set of measurements taken under the same conditions Accepted Value a quantity used by general agreement of the scientific community, a known value Example: 0°C freezing point of water or 100°C boiling point of water Experimental Value a quantitative value measured during an experiment Chapter 3 Vocabulary • Error • Percent Error • Significant Figures Error the difference between the accepted value and the experimental value Error = experimental value – accepted value Percent Error the percent that a measured value differs from the accepted value Percent error = |error | ____________ accepted value x 100% Significant Figures all the digits that can be known precisely in a measurement, plus a last estimated digit Chapter 3 Vocabulary • Mass • Weight • Volume Mass a measure of the amount of matter that an object contains SI base unit of mass is the kilogram Weight a force that measures the pull of gravity on a given mass Volume a measure of the space occupied by a sample of matter