Using Scientific Measurements
advertisement

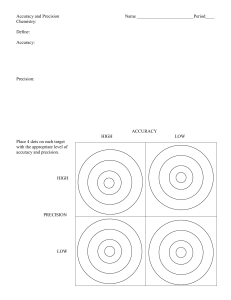
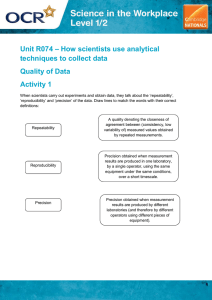
Using Scientific Measurements Uncertainty in Measurements All measurements have uncertainty. 1. Measurements involve estimation by the person making the measurement. 2. Measuring devices are limited in their precision Accuracy and Precision Accuracy – the closeness of measured values to the true or accepted value Precision – the closeness of repeated measurements of the same quantity Percent Error • Used to compare measured values to the true or accepted value. Measured Value Accepted value x100% accepted value Percent Error Sample Problems 1. Calculate the percent error in a length measurement of 4.25 cm if the correct value is 4.08 cm. 2. The actual density of a certain material is 7.44 g/cm3. A student measures the density of the same material as 7.30 g/cm3. What is the percent error of the measurement Counting Significant Figures Determine the number of significant figures in each of the following numbers: 0.003042 50.0 1.4030 10.47020 1000 250. 1000. 65,321 1000.00 2.00 x 102 0.060 1.004 x 105 Rounding to Significant Figures Round each of the following numbers to three significant digits: 1,566,311 2.651 x 10-3 84,592 0.0011672 0.07759 Calculations with significant figures 1. Find the volume of a cube that is 3.23 cm on each edge. 2. What is the sum of 67.14 kg and 8.2 kg? 3. Calculate the density of a 17.982 g object that occupies 4.13 cm3.