File - Chemistry 11 Enriched
advertisement

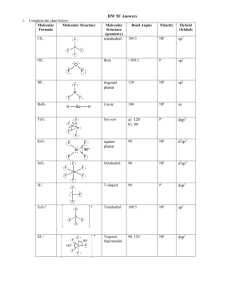
Chemistry 11 Enriched p. 341-348 Unit VII Problem Set 7.6 - Hybridization We have seen how the quantum picture leads to different shapes for s, p, d and f subshells. The question is how do these orbitals lead to bonding? For example, a bond may form from some s and some p electrons, but since the p electrons are in orbitals at 90 degrees to each other how does this work for bonds that are at 109 degrees? The answer to the question lies in the mashing together of these s and p orbitals in order to come up with new orbitals. These new orbitals are called hybrid orbitals. For each s or p put into the mix, one hybrid must be made. Consider CH4. The Lewis diagram looks like: H H C H H If we look closely at the outside shell of C we see that there are 2 2s electrons and 2 2p electrons. The s electrons are in a spherical arrangement and the p electrons are in lobes as pictured above. This does not connect to the 109.5 o we expect for the bonds that form. Since these 2s and 2p electrons are the ones used in bonding, we must resolve this issue. In order to explain it, we take these 4 orbitals (1 2s orbital and 3 2p orbitals – the 2px the 2py and the 2pz) and make 4 hybrid orbitals that will be called all be called sp3. The name comes from the fact that the new orbitals are a mashing (hybridizing) of 1 2s orbital and 3 2p orbitals. In short, whenever a molecule is tetrahedral with 4 bonds, it must be sp3 hybridized. The 4 bonds mean there must have been 4 parts put in (the s and the 3 p’s). In fact for any central atom with four repelling items – bonds or non bonding pairs, the hybridization will be sp3. So the oxygen on water, for example, is sp3, since there are two bonds and two non bonding pairs on the oxygen. Electron Configuration for C Before Bonding Electron Configuration for C After Bonding 2p PE PE sp3 2s All of the unpaired sp3 hybrid orbitals now hold one electron. These 4 equal orbitals repel each other so that they are all 109o apart, in a tetrahedral shape. Each one is shared with the one electron on a H atom to create the C-H bonds. If a molecule is trigonal planer (three bonds) it must have come from an s orbital and 2 p orbitals and so is sp2 hybridized. Any atom with three repelling items will be sp2 hybridized. Chemistry 11 Enriched p. 341-348 Unit VII Consider the H2CO molecule: H C=O H Electron Configuration for C Before Bonding Electron Configuration for C After Bonding 2p PE 2p sp2 hybrid PE 2s The three hybrid orbitals occupy the three equatorial positions that are 120o apart, and the unchanged p orbital still has its two lobes above and below the three hybrid orbitals – in the axial positions. half filled hybrid orbitals unhybridized p orbitals Each of the three hybrid orbitals can hold 2 electrons each and the p orbital system (both lobes above and below) can also hold two electrons. In a similar way, consider the oxygen molecule in the H2CO molecule which also is sp2 hybridized: Electron Configuration for O Before Bonding Electron Configuration for O After Bonding 2p PE 2p PE 2s sp2 hybrid Chemistry 11 Enriched p. 341-348 Unit VII Unhybridized p orbitals partly filled hybrid orbital Filled hybrid orbitals So, for the C and O together, the first bond is a sp2 – sp2 hybrid orbital overlap: The second bond occurs due to unhybridized p orbital overlap the electron density of the p system exists above and below the C and O nuclei, but TOGETHER, these two overlaps create one bond. Two H atoms bonded to the C sp2 hybrid orbitals Non-bonding hybrid orbitals on O Pi bond from unhybridized p orbital overlap Any hybrid orbital overalp is called a sigma bond. Any non-hybridized p orbital overalp is called a pi bond. In any multi bond system, there is always one sigma bond and one (or more) pi bonds. If a molecule is linear (two bonds) it must have come from an s orbital and a p orbital and is sp hybridized. This diagram is the bonding in ethyne, which has a triple bond between C atoms. The first is an sp – sp overlap, the second and third are unhybridized p orbital overlaps (pi bonds) Chemistry 11 Enriched p. 341-348 Unit VII Chemistry 11 Enriched p. 341-348 Unit VII The following chart summarizes all hybridizations. Summary of bond angles, shape names and Hybridization items repelling bonds lone pairs bond angle ( o ) name Hybridization of central atom example 2 2 0 180 linear sp H-C=C-H 3 0 trigonal planer BF3 3 120 sp2 2 1 bent N=O H 4 0 109.5 tetrahedral 3 1 ~107 trigonal pyramid 2 2 ~104 bent CH4 sp3 NH3 4 H2O Chemistry 11 Enriched p. 341-348 items repelling Unit VII bonds lone pairs bond angle ( o ) name 5 0 90 and 120 trigonal bipyramidal PF5 4 1 90 and 120 see saw SCl4 Hybridization of central atom dsp3 5 6 example 3 2 90 T-Shaped IF3 2 3 180 Linear XeF2 6 0 90 Octahedral SF6 5 1 90 Square Pyramidal 4 2 90 Square Planer d2sp3 ClF5 XeF4 Complete p. 348 Practice Problems #1,2 and p. 360 #3,4,5a Question: Going back to problem in the VSEPR Theory problem set, list the hybridization for those structures which have a central atom. Answers: a) sp3 e) sp3 i) dsp3 b) sp2 f) sp3 (oxygen) sp2 (nitrogen) j) d2sp3 c) sp2 g) dsp3 k) d2sp3 d) sp2 (carbon) and sp3 (oxygen) h) d2sp3 l) dsp3