Lecture Set No. 2
advertisement

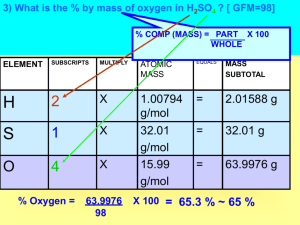
Material Balances Definitions Process - an operation or series of operations that causes a physical or chemical change thereby converting raw materials into products. Chemical Engineering Examples: reactors, mixers, separators, biological systems, etc. Balance - an accounting or inventory of mass and changes. System – an arbitrary portion or whole of a process as specified by the engineer analyzing the problem C A Mix B RP MX React Separate W P Accumulation = Input – Output + Generation - Consumption Steady State: Accumulation = 0 variables such as T, r, volume, flow rates etc. are not a function of time. Unsteady State (transient): At least one variable is a function of time. No reaction / conversion: Generation and consumption = 0 Example - New York City Input = People moving in Generation = Consumption = Accumulation = Output = Births Deaths Change in population People moving out Batch Process – heating water in a beaker Fixed amount of material Integral balance – changes summed over time Continuous Process – heating water flowing in pipes Continuous flowing streams in a pipe Differential balance – analysis at an instant time Semi-Batch Process – drain pot while heating Example– drug production process options Batch Continuous Semi-batch Steady state Transient Example Bioremediation is a method of cleaning up contaminated groundwater and soil. If a dilute solution of nutrients is pumped via a well into a closed soil layer underground at the rate of 1.5 kg/hr, and a recovery well removes 1.2 kg of depleted solution per hour, answer the following questions: a. What is the system (draw a picture)? b. What is the value of the input per hour? c. What is the value of the output per hour? d. What is the value of the accumulation per hour? e. What assumption has to be made to answer (d)? Reaction Balances CO2, CO, O2 CO2, CO, O2 2 CO + O2 = 2 CO2 In Total (kg/hr) Total Mass Out Total (kg/hr) kg CO /hr kg O2 /hr kg CO2 /hr Molecular Species kg CO /hr kg O2 /hr kg CO2 /hr kg C /hr kg O /hr Atomic Species kg C /hr kg O /hr Example Naphtha 250,000 ton /yr Steam Cracker CH4, H2 (39,500 T/yr) Fuel Gas Ethylene (55,000 T/yr) Polyethylene (30,000 T/yr) C2= Reactions Polystyrene (5,000 T/yr) PVC (40,000 T/yr) Propylene (45,000 T/yr) Acryonitrile (20,000 T/yr) C3= Reactions Dodecylbenzene (8,000 T/yr) Phenol / acetone (15,750 T/yr) Butene (30,700 T/yr) Synthetic rubber (10,000 T/yr) C4= Reactions Butenes (24,000 T/yr) Aromatics (68,000 T/yr) Aromatics Reactions Aromatics (48,000 T/yr) Fuel Oil (3,800 T/yr) Heavy Oil Degree of Freedom Analysis A stream of humid air enters a condenser in which 95 % of the water vapor in the air is condensed. The flow rate of the condensate (the liquid leaving the condenser) is measured and found to be 225 L/hr. Dry air may be taken to contain 21 mol % O2 and 79 mol % N2. Calculate the flow rate of the gas stream leaving the condenser and the mole fractions of oxygen, nitrogen, and water in this stream. F A Process N1 (mole dry air / hr) 0.21 mol O2 / mol 0.79 mol N2 / mol N2 (mol H2O / hr) N4 (mol O2 / hr) N5 (mol N2 / hr) N6 (mol H2O / hr) w 225 L H2O/hr N3 (mol H2O / hr) 95 % of water in feed Unknowns: N1, N2, N3, N4, N5, N6 Givens: (1-3) Material balances (4) Volume to molar conversion for Stream W (5) 95% water specification Degrees of freedom: 6-5 = 1 problem is underspecified Add one specification: Entering stream is 10 mol % H2O Problem Solving Procedure 1. READ and UNDERSTAND the problem statement. • Ask yourself • What information am I given? • What am I asked to do? • What other info might I need to solve the problem? 2. Select a BASIS. • The first two questions in Step 1 should help you pick an amount or a flow rate to use as the BASIS. • or assume an amount or flow rate (typically a multiple of 10) 3. DRAW and LABEL a process diagram • boxes (processes) and arrows (input & output) • Labels must include units. 4. ASSIGN ALGEBRAIC SYMBOLS to represent any unknowns using “Let x represent .....” statements. • Use as few unknowns / symbols as possible • Place symbols with units on process diagram. 5. COLLECT and TABULATE any additional data that may be required. 6. WRITE and BALANCE stoichiometric equations. 7. Create a TABLE OF BALANCES and UNKNOWNS 8. WRITE MASS BALANCES • Always start with A = I + G – O – C and cancel unnecessary terms with justification. • Balances must be independent. • Write the balances in order, starting with the balance with the fewest unknowns. • Number of independent balances should equal the number of unknowns. If not, look for other relationships between unknowns. • for non-reactive systems, the max number of independent balances = number of molecular species 9. SOLVE BALANCES / EQUATIONS 10. CHECK ANSWER 11. ANSWER THE FOLLOWING QUESTION: What did I learn? Example A solution composed of 50 wt% ethanol (EtOH), 10 wt% methanol (MeOH), and 40 wt% water (H2O) is fed at the rate of 100 kg/hr into a separator that produces one stream at the rate of 60 kg/hr with the composition of 80 wt% EtOH, 15 wt% MeOH, and 5 wt% H2O, and a second stream of unknown composition. Calculate the composition (in wt%) of the three compounds in the unknown stream and its flowrate in kg/hr. Material Balances Without Reactions Example 1000 kg of FeCl 3 ·6 H2O are added to a mixture of crystals of FeCl3 ·H2O to produce a mixture of FeCl3 ·2.5 H2O crystals. How much FeCl3 ·H2O must be added to produce the most FeCl3 ·2.5 H2O ? 10 Minute Problem A laundry can purchase soap (the desired material) containing 30.0 wt % water at a price of $ 7.00 /kg. The same manufacturer offers a soap containing 5 wt % water. If the freight rate is $ 6.00 / 100 kg of soap solution, what is the maximum price the laundry should pay the manufacturer for the soap containing 5.0 wt % water ? Note that the buyer has to pay the freight cost. Extra Practice Problems Problem Set Handout: II-1 – II-53 Material Balances Involving Multiple Units Example A) B) C) D) E) Write material balance equations around each block Calculate the degree of freedom analysis for each block Write the overall balance equations for the combined system Perform a degree of freedom analysis for the combined system Solve the equations using software on the Textbook CD P1 Feed = 100 kg wt % A 4 B 36 C 60 P2 W1 1 wt % A 15 B 30 C 55 wt % A 60 B 20 C 20 2 W2 = 20 kg wt % A 3.0 B ?? C ?? 10 Minute Problem A triple effect evaporator is designed to reduce water from an incoming brine (NaCl + H2O) stream from 25 wt % to 3 wt %. If the evaporator unit is to produce 14,670 lb/hr of NaCl (along with 3 wt % H2O), determine: a. the feed rate of brine in lb/hr. b. the water removed from the brine in each evaporator. Additional data are shown in the figure below V1 F Mass fraction H2O 1.00 V2 Mass fraction H2O 1.00 Mass fraction NaCl 0.25 H2O 0.75 V3 Mass fraction H2O 1.00 3 1 2 P1 Mass fraction NaCl 0.33 H2O 0.67 P2 Mass fraction NaCl 0.50 H2O 0.50 P3 14,670 lb/hr Mass fraction NaCl 0.97 H2O 0.03 The Chemical Reaction Equation and Stoichiometry Stoichiometry Definitions Stoichiometry: • theory of the proportions in which chemical species combine with one another in a chemical reaction as represented by a reaction equation. Stoichiometric Equation: • statement of the relative number of molecules or moles of reactants and products that participate in the reaction. • relates molecules, atoms, or moles but not mass. Stoichiometric Coefficients: numbers preceding each species in the balanced reaction equation. Stoichiometric ratio: ratio of stoichiometric coefficients of any two species. Example Gypsum (plaster of Paris : CaSO4 · 2H2O) is produced by the reaction of calcium carbonate and sulfuric acid (fed as a 98 wt% solution). A certain lime stone analyzes: CaCO3 96.89 %; MgCO3 1.41 %; inerts 1.70 %. For 5 metric tons (5000 kg) of limestone reacted completely, determine: The complete mass balance for all components entering and leaving the reactor. (MW : CaCO3 100.1; MgCO3 84.32; H2SO4 98; CaSO4 136; MgSO4 120; H2O 18; CO2 44) HINT: There are two reactions involved Important Definitions Limiting Reactant: RL Reactant that would disappear first if a reaction proceeded to completion. A reactant is limiting if it is present in less than its stoichiometric proportion relative to all other reactants and therefore used up first. The excess reactant is “left over”. Fractional Excess: f xs moles of i fed – moles of i req' d by stoichiome try for completion moles of i req' d by stoichiome try for completion where i represents the excess reactant Important Definitions Let RL represent the limiting reactant Conversion Let R represent a specified reactant – if no reactant is specified, assume RL is to be used Selectivity Let PD represent desired product moles R reacted moles of R fed moles PD in output moles of PunD in output Let PunD represent undesired products Let P represent products Extent of reaction Where: Yield ni nio vi extent of reaction ni = moles after reaction nio = moles before reaction vi = stoichiometric coefficient moles of P in output moles R fed Yield of PD moles of PD in output moles R fed Degree of completion Efficiency moles RL reacted moles of RL fed moles of PD in output moles of R reacted Example Limiting Reactants / Selectivity 40 Guys (25 Nerds, 15 Jocks) 30 Women (RL) Overall Reaction: Guys + Women → Couples (P) Desired Reaction: Nerds + Women → Couples (PD) Undesired Reaction: Jocks + Women → Couples (PunD) Prom Example In a process for the manufacture of chlorine by direct oxidation of HCL with air over a catalyst to form CL2 and H2O (only), the exit product is composed of HCL (4.4 mol%), CL2 (19.8 mol %), H2O (19.8 mol%), O2 (4.0 mol %) and N2 (52.0 mol%) 4 HCl + O2 → 2 Cl2 + 2 H2O What was: a) The limiting reactant (RL) b) The percent excess reactant c) The degree of completion of the reaction d) The extent of the reaction for HCl and O2 e) The conversion of both HCL and O2 10 Minute Problem The synthesis of ammonia proceeds according to the following reaction N2 + 3 H2 → 2 NH3 In a given plant, 4202 lb of nitrogen and 1046 lb of hydrogen are fed to the synthesis reactor per hour. Production of pure ammonia from this reactor is 3060 lb per hour. a. What is the limiting reactant. b. What is the percent excess reactant. c. What is the percent conversion obtained (based on the limiting reactant). Extra Practice Problems Problem Set Handout: II-54 – II-63 Material Balances for Processes Involving Chemical Reaction + Air (O2) H2O + N2 + CO2 Carbon (Coal) ENERGY Combustion Process Combustion Process → burning or oxidation of fuel to release energy • fuel usually contains C, H, & S • examples: coal, fuel oil, natural gas (methane), liquefied petroleum gas (propane & butane) • two types of combustion reaction: 1. “incomplete” – some of the C is converted to CO 2. “complete” – no CO is formed • all C goes to CO2 • all H goes to H2O • all S goes to SO2 Consider the following equations: • incomplete combustion of C • complete combustion of C • incomplete combustion of propane • complete combustion of propane 2 C + O2 → 2 CO C + O2 → CO2 2 C3H8 + 7 O2 → 6 CO + 8 H2O C3H8 + 5 O2 → 3 CO2 + 4 H2O • even if both reactions occur in the same process, write the two reactions separately ... do not combine. • Usual source of O2 is air: 21 mole-% O2, 79 % N2 • product gas is called “stack gas” or “flue gas” • stack gas is monitored in two ways: 1. Volume of gas produced 2. Chemical analysis of the stack gas – Orsat Analysis (dry basis) • gas leaving the reactor contains all products including H2O vapor • a sample is cooled to room temperature for chemical analysis ..... H2O condenses • thus, chemical analysis gives analysis on DRY BASIS • to know real WET BASIS composition of the stack gas, add back in the H2O Example: A gas with 40% A, 40% B, and 20% H2O has a Orsat dry gas analysis of 50% A and 50% B. • it is common to increase the amount of one reactant in order to (i) shift the equilibrium, and (ii) increase the conversion of the more expensive reactant • in combustion, the cheapest, but not free, component is air (O2) Definitions (combustion example) Theoretical Reactant (O2): moles of reactant (O2) required by stoichiometry for COMPLETE consumption of all the primary reactant to desired product (i.e. fuel to CO2 and H2O for combustion of a hydrocarbon). Theoretical Air: quantity of air that contains theoretical O2 Excess Reactant (O2): amount of reactant (O2) in excess of that required for COMPLETE reaction (combustion). Combustion Example A furnace used to provide heat to anneal steel burns a fuel oil whose composition can be represented as (CH2)n. It is planned to burn this fuel with stoichiometric air. a. Assume complete combustion and calculate the Orsat analysis of the flue gas. b. Recalculate the Orsat analysis assuming that 5 % of the carbon in the fuel burns to CO only (the O2 feed rate is the same as in Part a) Element Balance Example Ethanol (CH3CH2OH) undergoes a oxidative (air is fed to the reactor) dehydrogenation reaction to produce acetaldehyde (CH3CHO). Multiple side reactions occur in addition to the primary reaction. The reactor product stream is separated into an acetaldehyde product stream and a gas stream which contains water and the following components obtained from an Orsat analysis. Determine the flow rate, in kg, of the product streams based on a feed basis of 100 kg of ethanol. Orsat Anaylsis (mole %) CO2 O2 CO H2 CH4 N2 0.7 2.1 2.3 7.1 2.6 85.2 Polymath Solution POLYMATH Results 10-12-2009, Rev5.1.233 LEQ SOLUTION [1] x1 = 46.9 [2] x2 = 44.1 [3] x3 = 40.2 (F) (B) (W) LEQ REPORT Coefficients matrix and beta matrix x1 x2 x3 2 -2 0 | 5.6 3 -2 -1 | 12.3 0.5 -0.5 -0.5 | -18.7 The equations [1] 2·x1 - 2·x2 = 5.6 [2] 3·x1 - 2·x2 - x3 = 12.3 [3] 0.5·x1 - 0.5·x2 - 0.5·x3 = -18.7 General Number of equations : 3 Bio-Example Problem Glucose (C6H12O6) and ammonia (NH3) form a sterile solution (no live cells) fed continuously into a vessel containing a microorganism. Assume complete bioreaction. One product formed from the reaction contains ethanol, cells (CH1.8O0.5N0.2 ) and water. (The gas produced is CO2 ). If the reaction occurs anerobically (without the presence of oxygen) what is the minimum amount of kg of feed (glucose and ammonia) required to produce 4.6 kg of ethanol ? Only 60 percent of the moles of glucose are converted to ethanol. The remainder is converted to cell mass, carbon dioxide, and water. Polymath Solution POLYMATH Results 10-05-2009, Rev5.1.233 LEQ SOLUTION [1] x1 = [2] x2 = [3] x3 = [4] x4 = 0.8 4 0.8 1.8 LEQ REPORT Coefficients matrix and beta matrix x1 x2 x3 x4 0 1 1 0 | 4.8 -3 1.8 0 2 | 8.4 0 0.5 2 1 | 5.4 -1 0.2 0 0 | 0 The equations [1] x2 + x3 = 4.8 [2] -3·x1 + 1.8·x2 + 2·x4 = 8.4 [3] 0.5·x2 + 2·x3 + x4 = 5.4 [4] -x1 + 0.2·x2 = 0 General Number of equations : 4 Extra Practice Problems Problem Set Handout: II-69 – II-105 Material Balances Involving Chemical Reaction and Multiple Units Example Plants in Europe sometimes use the mineral pyrites (the desired compound in the pyrites is FeS2) as a source of SO2 for the production of sulfite pulping liquor. Pyrite rock containing 48.0 wt % sulfur, 43.0 wt % iron, and 9.0 wt % inerts is burned completely by flash combustion. All of the iron forms Fe3O4 in the cinder (the solids) and a negligible amount of SO3 occurs in either the cinder or the product gas. The exit gas from the absorber analyzes: SO2 0.7 mol %, O2 2.9 mol % and N2 96.4 mol %. Calculate the kg of air supplied to the burner per kg of the pyrites burned. (MW : S 32; Fe 56; O 16; N 14) Gas Pyrites Burner Sep 1 Sep 2 Solids Air SO2 Extra Practice Problems Problem Set Handout: II-106 – II-109 Recycle, Bypass, and Purge VAPRECYC PURGE SPLIT2 VAP COLUMN HEATEX MIXER H2 FLASH REACT MIXED OVHD RXNFEED RXNOUT BENZENE COLFEED BOT LIQ SPLIT1 LIQRECYC AspenPlus Cyclohexane Process Simulation Recycle is an example of a multi-unit system • Most often used with reactive system but our first example will be non-reactive • used in reactive systems to feed un-reacted reactants back into a reactor thereby achieving a higher conversion of expensive reactants. Bypass is also an example of a multi-unit system • used in both reactive and non-reactive systems Purge is also an example of a multi-unit system • used to eliminate the buildup of undesirable material in process Review Cyclohexane process diagram Guidelines / Definitions OVERALL & REACTOR Balances involve reaction. All others do not involve reactions. • always identify process, species, and type of balance • Reactor C2H4 Mole Balance: A = I – O + G – C Add the following to existing terminology: Overall Conversion of A N A input to the process N Aoutput from the process N A input to the process Single Pass Conversion of A N A input to reactor N Aoutput from reactor N A input to reactor Non-Reactive Example The flow chart of a process to recover crystalline potassium chromate (K2CrO4) from an aqueous solution of this salt is shown below. Forty-five hundred kilograms per hour of a feed solution that is 33.3 wt % K2CrO4 is joined by a recycle stream containing 36.36 wt % K2CrO4, and the combined stream is fed into an evaporator. The concentrated stream that leaves the evaporator contains 49.4 wt % K2CrO4. This stream is fed into a crystallizer in which it is cooled (causing crystals of K2CrO4 to come out of solution) and then filtered. The filter cake consists of K2CrO4 crystals and a solution that contains 36.36 wt% K2CrO4. The crystals account for 95 % of the total mass of the filter cake. The solution that passes through the filter, also 36.36 wt % K2CrO4, is the recycle stream. Calculate the kg/hr of water removed in the evaporator, the rate of production of crystalline K2CrO4 in kg/hr, the ratio (kg recycle) / (kg fresh feed) ,and the feed rates that the evaporator and crystallizer must be designed to handle in kg/hr. Water (Qw) Fresh Feed (F) Fe Filter Cake (Pc) Concentrate (Fc) Crystallizer / filter Evaporator Recycle (R) Solution (Ps) Reactive Example Hydrogen is used to reduce Fe2O3 to metallic iron (Fe) as shown below The reaction is: Fe2O3 + 3 H2 → 2Fe + 3 H2O Single pass conversion of Fe2O3 is 60 %. Determine: a) The overall conversion of Fe2O3 b) R in moles / hr c) Moles / hr of each component in the purge stream. : Purge – 0 % water Gases Condenser FF - 60 mol/hr H2 2 mol/hr CO2 GF Reactor W -100 % water F - 14 mol/hr Fe2O3 Separator R 80 mol % Fe2O3 20 mol % Fe Product – 24 mol/hr Fe 2 mol/hr Fe2O3 Reactive Example Consider the simple recycle operation shown below. Ethylene oxide is produced by the reaction 2CH2 =CH2 + O2 → 2CH2 CH2 O. Assume the separator is perfect; that is, it separates all unreacted ethylene and oxygen from the product ethylene oxide. The once-through or single-pass conversion is 50 %. The reactants in the fresh feed (FF) stream are in a 2:1 ratio. All unreacted reactants are recycled in the RC stream to join with the FF stream and form the mixed feed (MF) stream. Under these conditions, what is the recycle ratio required to produce and overall conversion of 100 %? Recycle ratio is defined as total moles of recycle (RC) per mole of P. Reconsider the above problem but this time assume the separator has been taken out of service; that is, the reactor product (RP) stream is simply split so that some of it is recycled (RC) and some of it is collected as product stream (P). Under these conditions, what recycle ratio is required to achieve an overall conversion of 75 percent? FF MF RP Reactor P Separator RC 10 Minute Problem Consider a more realistic process for the production of ethylene oxide. In this case air rather than pure oxygen is mixed with the ethylene gas to form the FF stream. The ethylene/oxygen ratio that results is not the stoichiometric 2:1. The separator (an absorber) is used as shown to the right. The separator product stream (SP) is split: some of it becomes the RC stream and some of it becomes the waste stream (W). The ethylene oxide product is removed from the bottom of the absorber as the P stream. Assume the separator is ideal as in the example problem. In this example the ethylene/air ratio being fed to the process is 1:10. The conversion of ethylene to ethylene oxide on a once-through basis is 55 %. What will be the overall conversion if 65 % of the gases leaving the absorber as SP are recycled? Reconsider the problem above from another point of view. Assume the separator is ideal. In this case, we analyze the W stream and find that it is 81.5 mole % N2 , 16.5 % O2 , and 2 % ethylene. We know that the recycle ratio RC/W is 3.0 in this problem. Calculate (a.) the ethylene/air ratio in the FF stream, and (b.) the conversion on a oncethrough basis. P FF RP MF Reactor SP Absorber RC W Extra Practice Problems Problem Set Handout: II-110 – II-135