Organic1 - Making Aspirin
advertisement

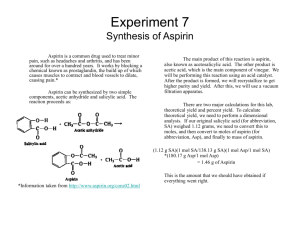
Organic Name: ____________________________________Page 1 of 1 Making Aspirin (Formal Lab Report) PURPOSE: To make aspirin (an aromatic compound), and to determine the purity of the aspirin by doing a melting point determination. PROCEDURE: Group 2 double or half the amounts and repeat. This may take 3 class periods. 1. Weigh out approximately two grams of salicylic acid. Be sure to record the exact mass of the salicylic acid. Add 5 mL of acetic anhydride (excess reagent) and 5 drops of concentrated H2SO4 Concentrated Sulfuric acid is very dangerous. Ask for help. The acid is in the black cabinet. Swirl until dissolved. 2, Heat using the water bath (pictured below) until the solid dissolves and the release of vapors stops. (Include a (Diagram) picture of the water bath set-up in your procedure section.) 3. Add 20 rnL of distilled water and let the mixture cool for 5 min. 4. Cool the mixture in an ice bath, and scratch the sides of the beaker with a stirring rod so that the aspirin will crystallize. Continue cooling until there is complete crystallization. 5. Weigh a piece of filter paper. 6. Filter the mixture to collect the aspirin. Use a wash bottle to wash all of the aspirin out of the beaker into the Buchner funnel. Set the filter paper with aspirin on a paper towel to dry overnight. When the liquid has gone from the filter paper rinse the aspirin twice with distilled water. 7. Calculate the theoretical yield of aspirin. 8. After the aspirin is dry weigh it on the filter paper, and calculate your percentage yield. 9. Calculate the Theoretical Yield of the Aspirin. Calculate the Percent Yield. 11. Test the pH of your aspirin. 12. Write a formal lab report. (Data Table, Analysis, Conclusion) Answer these questions in the conclusion of your lab report. You may do a podcast of the conclusions.: 1. Who discovered aspirin, what lead to this discovery, and what does this mean to us today? On a separate sheet. 2. Is aspirin an acid or base? ______________ 3. Devise and describe a way to test the pH of aspirin. 4. Test commercial aspirin’s pH. ______________ 5. What was your Aspirin’s pH. ______________ 6. If the pH is different in # 4 and 5, hypothesize why it is the same or why it is not the same?