File
advertisement

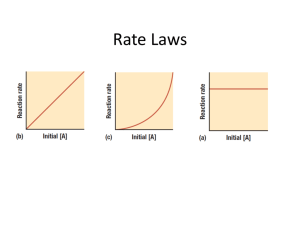
Unit 3: Kinetics ANTHONY GATES Chemical Kinetics The rate of reaction is defined as the amount of reactant converted into product over time The change in the concentration of a reactant or product over a unit of time. Rates [ A] at time t 2 [ A] at time t1 Rate t 2 t1 [ A] Rate t Reaction Rates… Can measure disappearance of reactants Can measure appearance of products Are proportional stoichiometrically Are equal to the slope of the tangent of any given point Change as the reaction proceeds, if the rate is dependent upon concentration [ NO2 ] constant t Rate Laws Differential rate law expresses how the rate depends on concentration. Typically called “the rate law.” Integrated rate law expresses how the concentrations depend on time. The Rate Law ∆[𝑁𝑂2 ] 𝑅𝑎𝑡𝑒 = = 𝑘[𝑁𝑂2 ]𝑛 ∆𝑡 k is a proportionality constant called the rate constant. n is the order of the reactant Has to be a whole number The sum of all of the powers of the reactants is the rate order. Both k and n must be determined by experiment. Rate Constant Important measurable quantity that characterizes the reaction. Vary over many order of magnitudes because reaction rates vary widely Method of Initial Rates The initial rate of a reaction is the instantaneous rate determined just after the reaction begins. Since the concentration of a product will influence the rate of a reaction, calculating the initial rate of reaction before much of the reactant has changed into product, allows us to circumvent the impact the concentration of the product will have on the rate of reaction, when calculating various rates in experiments. Investigation of data for initial rates enables prediction of how concentration will vary as the reaction progresses. Writing a (differential) Rate Law Problem - Write the rate law, determine the value of the rate constant, k, and the overall order for the following reaction: 2 NO(g) + Cl2(g) 2 NOCl(g) Experiment [NO] (mol/L) [Cl2] (mol/L) Rate Mol/L·s 1 0.250 0.250 1.43 x 10-6 2 0.500 0.250 5.72 x 10-6 3 0.250 0.500 2.86 x 10-6 4 0.500 0.500 11.4 x 10-6 Writing a Rate Law Part 1- Determine the values for the exponents in the rate law: R = k[NO]x [Cl2]y Looking at Experiment 1 and 2, [Cl2] is constant and as [NO] doubles, the rate quadruples. So the rate is second order with respect to [NO]. Quick math 0.250𝑚𝑜𝑙/𝐿 𝑛 0.250𝑚𝑜𝑙 𝑚 𝑘( ) ( ) 1.43 𝑥 10 𝑚𝑜𝑙/𝐿𝑠 𝐿 𝐿 = −6 0.500𝑚𝑜𝑙 𝑛 0.250𝑚𝑜𝑙 𝑚 5.72 𝑥 10 𝑚𝑜𝑙/𝐿𝑠 𝑘( ) ( ) 𝐿 𝐿 −6 0.25 = (0.500)𝑛 𝑛=2 Writing the Rate Law Part 1: Determine the values for the exponents in the rate law: R = k[NO]x [Cl2]y Looking at Experiment 2 and 4, [NO] is constant and as [Cl2] doubles, the rate doubles. So the rate is first order with respect to [Cl2]. Finishing it off… R = k[NO]2 [Cl2]1 The rate was determined to be second order according to [NO] and first order according to [Cl2]. 1.43 x 10-6mol/Ls = k (0.250mol/L)2 (0.250mol/L) k = 9.15 x 10-5 L2/mol2s Since the overall rate order is equal to the sum of the exponents, this reaction’s rate order is 3rd order. Graphing with your calculator L1: L2: 0; 120; 300; 600; 1200; 1800; 2400; 3000; 3600; 1.00; 0.91; 0.78; 0.59; 0.37; 0.22; 0.13; 0.082; 0.050 L3: 1/(L2) Graph L1 vs L2 Graph L1 vs L3 Integrated Rate Law Relating concentration to time The rate order (sum of the exponents) can be determined by graphing experimental data Zero First Order: Concentration vs. time is linear Order: ln[Concentration] vs. time is linear Second Order: 1/Concentration vs. time is linear Relationship of the Integrated Rate Law Concentration of A depends on time. As long as the initial concentration of A and the rate constant k are known, the concentration at any time t can be found. Zero Order … very rare circumstances 𝑅𝑎𝑡𝑒 = ∆[𝐴] ∆𝑡 = 𝑘[𝐴]0 = 𝑘 Integrated [A]= Rate Law: -kt + [A]0 Where [A0] [A] is the concentration at time t is the initial concentration at t=0 Zero Order cont. [A]= -kt + [A]0 is a form of y=mx+b, where… y=[A] m= -k x=t b= [A0] A reaction is zero order if and only if [A] vs. t produces a straight line. First Order 𝑅𝑎𝑡𝑒 = = 𝑘[𝐴] Integrated rate law: ln[A] ∆[𝐴] ∆𝑡 = -kt + ln[A0] ln[A] = -kt + ln[A0] is a form of y=mx+b, where… y= ln[A] m=-k x=t b= ln[A0] A reaction is first order if and only if ln[A] vs. t produces a straight line. First Order cont. This law can also be expressed as ln 𝐴0 𝐴 = 𝑘𝑡 Second Order ∆[𝐴] ∆𝑡 = 𝑘[𝐴]2 𝑅𝑎𝑡𝑒 = Integrated Rate Law: 𝟏 𝑨 = 𝒌𝒕 + 1 𝐴0 𝟏 𝑨𝟎 is a form of y=mx+b, where… 1 𝐴 = 𝑘𝑡 + y= 1/[A] A reaction is second order if and only if 1/[A] vs. t produces a straight line. m=k x=t b= 1/[A0] Solving an Integrated Rate Law Time (s) [H2O2] (mol/L) 0 1.00 120 0.91 300 0.78 600 0.59 1200 0.37 1800 0.22 2400 0.13 3000 0.082 3600 0.050 Problem: Find the integrated rate law and the value for the rate constant, k Time vs. [H2O2] Time (s) [H2O2] 0 1.00 120 0.91 300 0.78 600 0.59 1200 0.37 1800 0.22 2400 0.13 3000 0.082 3600 0.050 Time vs. ln[H2O2] Time (s) ln[H2O2] 0 0 120 -0.0943 300 -0.2485 600 -0.5276 1200 -0.9943 1800 -1.514 2400 -2.04 3000 -2.501 3600 -2.996 Time vs. 1/[H2O2] Time (s) 1/[H2O2] 0 1.00 120 1.0989 300 1.2821 600 1.6949 1200 2.7027 1800 4.5455 2400 7.6923 3000 12.195 3600 20.000 And the winner is… Time vs. ln[H2O2] 1. As a result, the reaction is 1st order 2. The (differential) rate law is: R k[ H 2O2 ] 3. The integrated rate law is: ln[ H 2O2 ] kt ln[ H 2 02 ]0 4. But…what is the rate constant, k ? Finding the Rate Constant, k Method #1: Calculate the slope from the Time vs. ln[H2O2] table. ln[ H 2O2 ] 2.996 slope t 3600 s 4 1 slope 8.32 x10 s Now remember: ln[ H 2O2 ] kt ln[ H 2 02 ]0 k = -slope k = 8.32 x 10-4s-1 Time (s) ln[H2O2] 0 0 120 -0.0943 300 -0.2485 600 -0.5276 1200 -0.9943 1800 -1.514 2400 -2.04 3000 -2.501 3600 -2.996 Finding the Rate Constant, k Method #2: Obtain k from the linear regression analysis. 4 slope 8.32 x10 s 1 Now remember: ln[ H 2O2 ] kt ln[ H 2 02 ]0 k = -slope k = 8.32 x 10-4s-1 Half Life The time required for a reactant to reach half of its original concentration. It does not matter what the original amount is… Half life is specific to each reactant. Half Life Cont. Zero Order: First Order: 𝑡1/2 = 𝐴0 2𝑘 𝑡1/2 = 0.693 𝑘 Second Order: 𝑡1/2 = 1 𝑘𝐴0 Pseudo-first order rate law If there are multiple reactants, you can write the first order rate law with respect to the concentration of one of the reactants and assume the others to be constants with values equal to their initial concentrations. The rate constant (k’) for the pseudo-first order rated law is not the rate constant (k) for the entire reaction, but solely with respect to the chosen reactant. Pseudo cont. Rate = k [A]n [B]m [C]p Can be reduced to: Rate = k’ [A]n To find the rate constant for the entire reaction: k’ = k [B]0m[C]0p When [B]0 k’ is the rate constant for the pseudo-first order rate law and [C]0 are any reactants not chosen. Rate Laws Summary Zero Order First Order Second Order Rate = k Rate = k[A] Rate = k[A]2 Integrated Rate Law [A] = -kt + [A]0 ln[A] = -kt + ln[A]0 1 1 kt [ A] [ A]0 Plot the produces a straight line [A] versus t ln[A] versus t 1 versus t [ A] Slope = -k Slope = -k Slope = k 0.693 t1/ 2 k 1 t1/ 2 k [ A]0 Rate Law Relationship of rate constant to slope of straight line Half-Life [ A]0 t1/ 2 2k Bell Work Sketch a straight line graph for zero order, first order and second order. Label the axis appropriately For each graph, relate the slope to “k” Pre-lecture task complete while waiting for the bell! Write down a description of the path you take to get from your class to lunch and what parts of your path cause you to go faster or slower. List any issues you can think of that prevents you from getting to eat your lunch sooner (ex. lines, hallway traffic, maze-like path, etc.) Learning Objective I can describe the relationship between a mechanism and its rate law. Reaction Mechanisms A + 3B AB3 Rate=k[A][B]2 A “reaction mechanism” is a series of steps by which the reaction occurs, and the steps can occur at different rates: Step 1 A + 2B AB2 (Slow) Step 2 AB2 + B AB3 (Fast) Overall A + 3B AB3 (must add up to overall reaction) Each step is called an “elementary step” AB2 is an “intermediate” – a substance that is part of the mechanism but doesn’t show up in the balanced equation Reaction Mechanism cont. A + 3B AB3 Rate=k[A][B]2 Step 1 A + 2B AB2 (Slow) Step 2 AB2 + B AB3 (Fast) Overall A + 3B AB3 The rate law for an elementary step can be written from the coefficients in the elementary step For Step 1 the rate law is: Rate=k[A][B]2 For Step 2 the rate law is: Rate=k[AB2][B] A little something to consider… Key Concept – the slow step is the rate determining step The rate of the slow step is the rate of the overall reaction Let’s try one out! Step 1 Step 2 Step 3 What would the overall reaction be (balanced equation)? Overall What would the rate law be?? Rate = k[A]2 What are A2, X and A2X? Intermediates!! Note – intermediates do not appear in the rate law 2AA2 A2 + XA2X A2X + B A2B + X (SLOW!) (Fast!) (Fast!) 2A + B A2B Your turn!! For the reaction 3X+2YX3Y2 the rate law is Rate=k[X]2[Y] The following mechanism is proposed: Step 1: X+YXY Step 2: 2X+YX2Y Step 3: XY+X2YX3Y2 Which is the rate limiting step? Step 2 What is (are) the intermediates? XY and X2Y Try this on for size!! For the reaction 2A + 2B A2B2 the rate law is Rate=k[A]2 Two proposed mechanisms are: Mechanism 1: Step 1 2A + B A2B Slow Step 2 A2B + BA2B2 Fast 2A A2 Slow Step 2 A2+BA2B Fast Step 3 A2B+BA2B2 Fast Mechanism 2: Step 1 Which mechanism is consistent with the rate law? For a hypothetical chemical reaction that has the stoichiometry 2 X + Y Z, the following initial rate data were obtained. All measurements were made at the same temperature. Initial Rate of Formation of Z, Initial [X]o, Initial [Y]o, (mol.L-1) (mol.L-1) (mol.L-1.sec-1) 7.010-4 0.20 0.10 -3 1.410 0.40 0.20 2.810-3 0.40 0.40 -3 4.210 0.60 0.60 (a) Give the rate law for this reaction from the data above. (b) Calculate the specific rate constant for this reaction and specify its units. (c) How long must the reaction proceed to produce a concentration of Z equal to 0.20 molar, if the initial reaction concentrations are [X]o = 0.80 molar, [Y]o = 0.60 molar and [Z]0 = 0 molar? (d) Select from the mechanisms below the one most consistent with the observed data, and explain your choice. In these mechanisms M and N are reaction intermediates. (1) X + Y M (slow) X+MZ (fast) (2) X + X M (fast) Y+MZ (slow) (3) Y M (slow) M+XN (fast) N+XZ (fast) Factors that Affect Reaction Rates Temperature Concentration (except in zero order rxns) Surface Area Other environmental factors Presence of other chemicals https://www.youtube.com/watch?v=OttRV5ykP7A Temperature Generally as the temperature increases, so does the reaction rate. The reaction constant (k) is temperature dependent. Concentration & Surface Area Other than a zero order… as you increase the concentration, the rate should increase. Increasing the amount of reactant present increases the likelihood of two reactants colliding and reacting. Increasing the surface area should increase the reaction rate. Increasing the amount of surface area present will allow for more of a reactant to make collisions without being shielded by more of that reactant. Collision Theory/Model In chemical reactions bonds are either broken or formed. Molecules must collide in order to react. More Collisions More Reactions Qualitatively explains the order to elementary reactions and the temperature dependence of the rate constant. Collision Theory/Model Molecules must collide with the correct orientation in space and with enough energy in order to cause the original bonds to break and for new bonds to form. Activation Energy The minimum amount of energy required for a reaction to occur is called the activation energy, Ea Just as a ball cannot go over a hill if it does not have sufficient energy, a reaction cannot occur unless the molecules possess sufficient energy to get over the activation energy barrier. Correct Orientation and Energy 2NOCl2NO + Cl2 Reaction Coordinate Diagrams We can see how the energy changes throughout a process on a reaction coordinate diagram. Reaction Coordinate Diagrams Shows the change in energy throughout the reaction (ΔE). The species present at the local maximum is called a transition state. The energy gap between the initial energy and that of the transition state is the activation energy. Unimolecular Reactions Unimolecular reactions occur because collisions with solvent or background molecules activate the molecule in a way that can be understood in terms of a Maxwell-Boltzmann thermal distribution of particle energies. Maxwell Boltzmann Diagrams Another way to “see” how energy (temperature) affects a reaction rate. Temperature is defined as a measure of the average kinetic energy of the molecules in a sample. At any temperature there is a wide distribution of kinetic energies. Maxwell Boltzmann Diagrams As the temperature increases, the curve flattens and broadens. Thus at higher temperatures, a larger population of molecules has higher energy. Maxwell Boltzmann Diagrams If the dotted line represents the activation energy, as the temperature increases, so does the fraction of molecules that can overcome the activation energy barrier. As a result, the reaction rate increases. Maxwell Boltzmann Diagrams The fraction of the molecules present in a gas which have energies equal to or in excess of the activation energy at a particular temperature can be found through the expression: where R is the gas constant and T is the temperature in Kelvin . Arrhenius Equation Svante Arrhenius developed a mathematical relationship between k and Ea: where A is the frequency factor, a number that represents the likelihood that collisions would occur with the proper orientation for reaction. reaction = the probability that any given collision will result in a Arrhenius Equation Taking the natural logarithm of both sides, the equation becomes… y = mx + b Arrhenius Equation k = number of collisions that result in a reaction per second A = the total number of collisions (leading to a reaction or not) per second Arrhenius Equation It can be seen that either increasing the temperature or decreasing the activation energy (for example through the use of catalysts) will result in an increase in rate of reaction. Thought provoking ideas… One generalization derived from the Arrhenius' equation is that, for many common chemical reactions at room temperature, a 10 degree Celsius increase in temperature doubles the reaction rate. Catalyst Catalysts can stabilize the transition state, lowering the activation energy and thus increasing the reaction rate. Catalysts can increase the reaction rate by participating in the formation of a new reaction intermediate, thereby providing a new reaction pathway or mechanism. Catalysts are present throughout the entire reaction and do not change concentration. Catalysis: types of catalysts In acid-base catalysis, a reactant either gains or loses a proton; this changes the rate of the reaction. In surface catalysis, either a new reaction intermediate is formed, or the probability of successful collisions is modified. Some enzymes accelerate reactions by binding to the reactants in a way that lowers the activation energy. Other enzymes react with the reactant species to form a new reaction intermediate. Enzymes Enzymes are catalysts in biological systems. The substrate fits into the active site of the enzyme much like a key fits into a lock.






![w_12_kinetics_graphical_determination_of_rate[1]](http://s3.studylib.net/store/data/008634121_1-13227467ad7c89de0b1e879c8a34752a-300x300.png)