w_12_kinetics_graphical_determination_of_rate[1]
advertisement
![w_12_kinetics_graphical_determination_of_rate[1]](http://s3.studylib.net/store/data/008634121_1-13227467ad7c89de0b1e879c8a34752a-768x994.png)
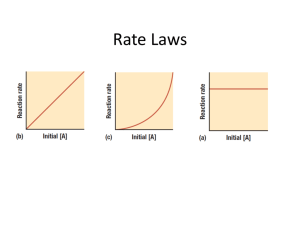
Kinetics Reaction Order Reaction Rates Rate Constant Obtain the Excel spreadsheet that goes with this activity The spreadsheet has two columns for you to enter data obtained from an experiment measuring the change of concentration over time. By analyzing the three graphs produced you can determine the order of the reaction and values for the rate constant (k) Blue Line is the graph specified in the title. The black line is a trend line (calculated by least squares analysis) The trend line is used to calculate the linear equation for the data selected. You will want to use the graph with the trend line that matches the graphed data-- the data line may be obscured by the trend line. If the trend line lies over the line of the data graphed is means the linear equation accurately matches the data graphed. The linear equation for the trend line is shown under the title of each graph. y=mx=b m=slope Reaction Order Summary---study this summary to understand how to graphically determine the order of a reaction. Zero Order Reactions Graphical characteristics A plot of concentration of reactant vs time results in a straight line with a negative slope k = -slope Rate = k Rate law for Zero Order Reactions (The rate of the reaction does not depend on the concentration or the reactants) First Order Reactions Graphical characteristics A plot of natural log of the concentration of reactant vs time results in a straight line with a negative slope k = -slope Rate law for First Order Reactions - ln [A]t = kt + ln [A]0 y = mx + b ---equation of a straight line (Concentration doubles—rate of reaction doubles) Second Order Reactions Graphical characteristics A plot of the reciprocal of the concentration ( 1/[A] ) of reactant vs time results in a straight line with a positive slope k = slope Rate law for second Order Reactions 1 1 kt [ A] t [ A] 0 y =mx + b ---equation of a straight line (Concentration doubles—rate of reaction doubles) Problem #1 graph decomposition of hydrogen peroxide 2 H2O2 2 H2O + O2 Identify order of reaction time What is value of k for reaction? 0 300 600 1200 1800 3000 [H2O2] 2.32 1.86 1.49 0.98 0.62 0.25 write the rate law for the reaction Problem #2 Graph reaction of Buckminsterfullerene C60O3 C60O + O2 Identify order of reaction What is value of k for reaction? Write the rate law for the reaction Problem #3 Solutions of A=H3COC6H4CNO in carbon tetrachloride dimerize slowly as shown by the following data Identify order of reaction What is value of k for reaction? Write the rate law for the reaction Time min 3 9 15 21 27 33 39 45 51 57 63 69 75 [C60O3] 0.04241 0.03634 0.03121 0.0268 0.02311 0.01992 0.01721 0.01484 0.01286 0.01106 0.00955 0.00827 0.0071 Time Hrs 0 3.5 7 10.5 14 17.5 21 24.5 28 31.5 35 H3COC6H4CNO 0.995 0.745 0.595 0.494 0.424 0.37 0.331 0.295 0.27 0.247 0.229 Problem #4 Identify order of reaction What is value of k for reaction? Write the rate law for the reaction Time Sec 0 1 2 3 4 5 6 [A] 8 7.33 6.66 5.99 5.332 4.665 3.998 Problem 1 Use the rate law to determine the time when half of the reactant has been used up Problem 2 Use the rate law to determine the time when half of the reactant has been used up Problem 3 Use the rate law to determine the time when half of the reactant has been used up Problem 4 Use the rate law to determine the time when half of the reactant has been used up