Atomic Theory and Bonding Outline December 2013
advertisement

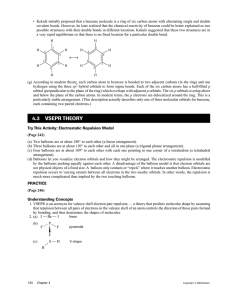
SCH4U Atomic Theory and Bonding December 2013 Assumed Prior Knowledge 1. Write electron configurations for atoms 2. Drawing Lewis and structural diagrams for covalent compounds 3. Use the VSEPR theory to predict the shapes: linear, v-shaped, trigonal planar, trigonal pyramid, tetrahedron 4. Using electronegativity values, predict the polarity of molecules 1. Atomic Theory: Quantum Mechanical Model a) BEFORE coming to class read p. 192-3 (Section 3.6) b) Review of Bohr-Rutherford diagrams and electron configuration c) Practice Questions: p. 194 #6-11 d) BEFORE coming to class read p.181-184 (Section 3.6) e) (Quantum Numbers) f) BEFORE coming to class read p. 199-201 (Section 3.7) g) Note: Orbitals and Orbital Diagrams h) Quiz #22 2. Covalent Bonding a) BEFORE coming to class read p. 231-234 (Section 4.2) b) Note: Valence Bond Theory c) Quiz #23 d) BEFORE coming to class read p. 236-241 (Section 4.2) e) Sigma and Pi Bonds f) BEFORE coming to class read p. 242-248 (Section 4.3) g) VSEPR Theory h) Activity: Making 3-D Molecules i) BEFORE coming to class read p. 251-256 (Section 4.4) j) Polar Molecules k) BEFORE coming to class read p. 257-262 (Section 4.5) Continue with your “Meaning of Terms” page. l) PowerPoint: Intermolecular Forces m) Practice Questions: page 260 #1-5 3. Organic Compounds Cont’d a) b) c) d) e) Alcohols and Ethers (section 1.5 p.38-45) Ethers (p.45-46) Aldehydes and Ketones ( p. 49-52) Carboxylic Acids (p.58-60) Intermolecular Forces and Properties of Organic Compounds 4. Review for Test a) Assignment: Review for Test b) Assignments: Self-Assessment and Practice Test c) Textbook Questions for Extra Practice: Self-Quiz p. 219 #1-7, 15, 16, 18; Chapter 3 Review p. #3, 12, 13, 15, 17; Self-Quiz p. 281 #1-8, 12-18; Chapter 4 Review p. 282 #2-4, 7-20, 22 d) See website for extra multiple choice questions