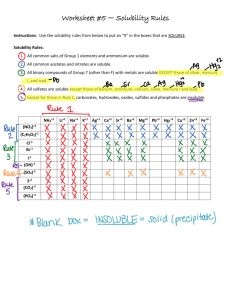
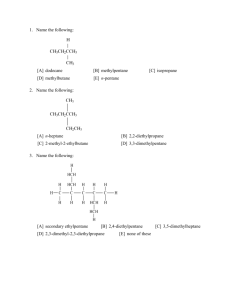
Play-Doh Lab Your Task: You will use Play-Doh to explore the shapes of common compounds. In the Lab: 1. Determine the Lewis structure for each of the following compounds OF2 CH4 N2 O2 SeCl6 AsF5 NH3 BF3 BeF2 CI4 CO2 AlH3 HF 2. Determine the shape of the molecule using VSEPR Theory. 3. Create a model of the molecule using Play-Doh and toothpicks. Use half a toothpick for each bond to help hold them together better and conserve toothpicks. 4. Using you knowledge of intermolecular forces and VSEPR Theory, predict what intermolecular forces these molecules would exert on another molecule of the same compound. 5. Create a data table including the following columns: Number of Valence Electrons, Lewis Structure, Number of Shared Pairs on the Central Atom, Number of Unshared Pairs on the Central Atom, Molecular Geometry, 3D Picture of the Model, and Intermolecular Forces for this Molecule. The columns italicized above do not need to be completed for nitrogen, oxygen, and hydrogen fluoride. 6. Please note that oxygen and nitrogen exist naturally as O2 and N2. When chemists refer to “oxygen” or “nitrogen,” we are referring to O2 and N2. Lab Report: A) Purpose B) Analysis Questions 1) Of the compounds above, which ones do you expect to be soluble in water? Justify your answers. 2) Of the compounds above, which ones do you expect to be soluble in carbon tetrachloride? Justify your answers. 3) Oxygen, nitrogen, and hydrogen fluoride all have similar geometries. Which of the three do you expect to have the highest boiling point? Justify your answer. 4) Based on its intermolecular forces, you would not expect carbon dioxide to be soluble in water. For example, the solubility of oxygen in water at 25°C and atmospheric pressure is 8.3mg/L. However, carbon dioxide is fairly soluble in water and has a solubility of 1.45g/L at atmospheric pressure. Explain why you would not have predicted carbon dioxide’s higher solubility in water. Then research the reason why carbon dioxide is fairly soluble in water. Be sure to cite your sources. C) Conclusions D) Chart of the 13 compounds