Carbohydrates
advertisement

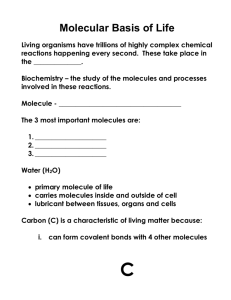
1. How is an element different from a compound? 2. What would be the pH of a neutral solution? acidic solution? basic solution? 3. What four elements are most often found in organic compounds? 4. Covalent bonds are formed by the sharing of electrons. How many covalent bonds can a carbon atom form? 5. Turn in Biological Molecule hand out from Yesterday Carbohydrates 1. Chemical Composition Carbohydrates contain atoms of carbon, hydrogen, and oxygen that are bonded together by the sharing of electrons (covalent bonds). Energy is stored in these bonds. The simplest carbohydrates are the simple sugars or monosaccharides. The most important monosaccharide for living things is glucose (molecular formula = C6H12O6). Nearly all living things use glucose as a source of energy. Please draw the structural formula for glucose in you class notes: 2. Sugars (carbohydrates) are biologically important because they contain large amounts of energy. The human body uses glucose for energy. Carbohydrates that are not immediately used by the body are stored as complex sugars known as starch. They are later broken down into simple sugars (glucose) when energy is needed. 3. Dietary Sources of Carbohydrates Carbohydrates (sugars) are obtained by eating vegetables, fruits, breads, cereals, rice, pasta, and sweets. Roughage, such as cellulose, is a special type of complex carbohydrate that cannot be digested by humans. 4. Carbohydrate Storage Unused carbohydrates are stored in the liver as a complex carbohydrate called glycogen. Check Your Understanding 1. What is the molecular formula for glucose? 2. If glucose is a monosaccharide, what does a disaccharide look like? Polysaccharide? 3. What are the functions of cellulose and glycogen?