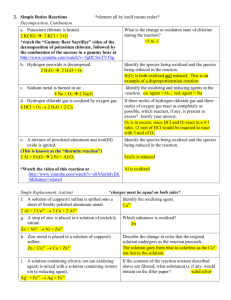
Chemistry 232
advertisement

Chemistry 232
Electrochemistry Notes
Electrochemical Cells
Galvanic cells –
an electrochemical cell that drives
electrons through an external circuit
spontaneous redox reaction occurring
inside cell.
The Zn/Cu Galvanic Cell
half-reactions
Cu2+ (aq) + 2 e- Cu (s) (cathode, RHS)
Zn2+ (aq) + 2 e- Zn (s) (anode, LHS)
A Schematic Galvanic Cell
e-
Porous Disk
e-
eReducing Agent
Anode
Oxidizing Agent
Cathode
The Zinc/Copper galvanic cell.
e-
Zn(s)
1.10 V
Porous Disk or
Salt Bridge
e-
Cu(s)
e-
ea(Zn2+) = 1.00
Anode
a(Cu2+) = 1.00
Cathode
Cell Reactions
The difference in the RHS and the LHS
reaction
Cu2+ (aq) + Zn (s) Cu (s) + Zn2+ (aq)
For each half reaction, we can write the
reaction quotient as follows
Cu2+ (aq) + 2 e- Cu (s)
Q = 1/ a(Cu2+)
Zn2+ (aq) + 2 e- Zn (s)
Q = 1/ a(Zn2+)
Overall Qcell = a(Zn2+) / a(Cu2+)
Cell Diagrams
A shorthand way of expressing what
takes place in an electrochemical
cell.
For the above electrochemical cell.
Pt Cu (s) Cu2+ (aq) Zn2+ (aq) Zn (s) Pt
Note
phase boundary
salt bridges
liquid junction
Another Example
The cell reaction
H2 (g) + Cu2+ (aq) 2 H+ (aq) + Cu
(s)
Pt H2 (g) H+ (aq) Cu2+ (aq) Cu (s) Pt
Electrochemical cells
a cell that has not reached equilibrium can
do electrical work by driving electrons
through an external wire.
Reversible Electrochemical Cells
In order for us to make measurements
on an electrochemical cell, it must be
operating reversibly.
Place an opposing source of potential in
the external circuit
Cell operates reversibly and at a constant
composition.
we,max = G
The Measurement of Cell
Potentials
Measure the potential of an electrochemical
cell when the cell is at equilibrium, i.e., the
state between the galvanic and the
electrolytic cell.
Counter potential
(load)
ePorous Disk
e-
eReducing Agent
Anode
Oxidizing Agent
Cathode
Derivation of the Nernst Equation
Consider an electrochemical cell that
approaches the equilibrium state by
an infinitesimal amount d
dG J J d rxn G d
J
Reminder
dG
r G J J
J
d
T ,P
The Work in Transporting Charge
The maximum work
dw e ,max rxn G d
For the passage d electrons from the
anode (LHS) to the cathode (RHS)
eN A d F d
F = Faraday’s constant = e NA = 96485 C/mole
The Cell Potential
The work to transport charge
dw e ,max F d E cell
dw e ,max
rxn G
d
rxn G
- E cell
F
Standard Cell Potentials
From the reaction Gibbs energy
r G r G o RT lnQcell F E cell
o
RT lnQcell
r G
r G
E cell
F
F
F
We define
E cell
r G
F
o
The Nernst Equation
E cell
RT
E
lnQcell
F
E represents the standard cell potential,
the potential of the cell when all cell
components are under standard
conditions.
f (all gases) = 1
a (solutes) = 1
T = 298.15 K
P = 1.00 bar pressure
Cells at Equilibrium
When the electrochemical cell has
reached equilibrium
E cell 0 V Qcell K cell
Kcell = the equilibrium constant for the cell reaction.
RT
FE
E
ln K cell ln K cell
F
RT
Knowing the E° value for the cell, we can estimate
the equilibrium constant for the cell reaction.
Equilibrium Constant Calculations
from Cell Potentials
Examine the following cell.
Pt Sn2+ (aq), Sn4+ (aq) Fe3+ (aq) Fe2+ (aq)
Pt
Half-cell reactions.
Sn4+ (aq) + 2 e- Sn2+ (aq) E(Sn4+/Sn2+) = 0.15 V
Fe3+ (aq) + e- Fe2+ (aq)
E (Fe3+/Fe2+) = 0.771 V
Cell Reaction
Sn2+ (aq) + 2 Fe3+ (aq) Sn4+ (aq) + 2 Fe2+ (aq)
Ecell = (0.771 - 0.15 V) = 0.62 V
Standard Reduction Potentials
Standard reduction potentials are
intensive properties.
We cannot measure the potential of
an individual half-cell!
We assign a particular cell as being
our reference cell
Assign values to other electrodes on
that basis.
The Standard Hydrogen Electrode
Eo (H+/H2) half-cell = 0.000 V
e-
f{H2(g)} = 1.00
H2 (g)
a (H+) = 1.00
Pt gauze
A Galvanic Cell With Zinc and the
Standard Hydrogen Electrode.
e-
0.763 V
e-
Porous Disk or
Salt Bridge
Zn(s)
a(Zn2+) = 1.00
Zn2+, SO42-
Anode
a (H+) = 1.00
Source of H+ (e.g.,
HCl (aq), H2SO4 (aq))
Cathode
H2 (g)
Pt gauze
The Cell Equation for the ZincStandard Hydrogen Electrode.
The cell reaction
2 H+ (aq) + Zn (s) H2 (g) + Zn2+ (aq)
Pt Zn (s) Zn2+ (aq),a=1 H+ (aq), a=1 H2 (g), f=1 Pt
When we measure the potential of this cell
Ecell = ERHS - ELHS
but ERHS = E(H+/H2) = 0.000 V
Ecell = E(Zn2+/Zn) = 0.763 V
The Spontaneous Direction of
a Cell Reaction
Examine the magnitude the of the
standard cell potential!
E cell
rxn G
F
o
If the standard cell potential is positive, the
rG is negative!
The Composition Dependence of the
Cell Potential
Nonstandard cell potential (Ecell) will be
a function of the activities of the
species in the cell reaction.
E cell
RT
E
lnQcell
F
To calculate Ecell, we must know the cell
reaction and the value of Qcell.
Example
For the following system
Pt H2 (g) H+ (aq) Cu2+ (aq) Cu (s) Pt
Calculate the value of the cell potential when
the f (H2) = 0.50, a(Cu2+) = 0.20, and a(H+) =
0.40.
Concentration Cells
Electrolyte concentration cell
the electrodes are identical; they simply
differ in the concentration of electrolyte in
the half-cells.
Concentration Cells (II)
Electrode concentration cells
the electrodes themselves have
different compositions. This may be
due to.
Different fugacities of gases involved in
electrode reactions (e.g., The H+ (aq)/H2
(g) electrode).
Different compositions of metal amalgams
in electrode materials.
Applications of Electrochemistry
Measurement of activities and activity
coefficients.
Electrochemical series.
Equilibrium constants and
thermodynamic functions of cell
reactions
Obtaining Standard Cell Potentials
Look at the following cell
Pt H2 (g) HCl (aq) AgCl (s) Ag (s) Pt
E cell E
cell
RT
a H a Cl
ln
F
f (H 2 )
Ecell = E(AgCl/Ag) - E (H+/H2)
= E(AgCl/Ag)
Ecell Values and Activity Coefficients
E cell
In dilute solution, using the DHLL
1
4.606 RT
2.344 RT
log m E (AgCl Ag )
m 2
F
F
Plot LHS vs. m1/2
Once Ecell is known, we can obtain
experimental estimates of the mean activity
coefficients.
The Calculation of Standard Cell
Potentials
0.236
0.234
0.232
0.230
0.228
y = 0.0582x + 0.2222
R2 = 0.9836
0.226
0.224
0.222
0.220
0.00
0.05
0.10
0.15
0.20
0.25
0.30
0.35
0.40
Electrochemical Series
Look at the following series of reactions
Cu2+ (aq) + 2 e- Cu (s)
Zn2+ (aq) + 2 e- Zn (s)
Zn has a thermodynamic tendency to
reduce Cu2+ (aq)
Pb2+ (aq) + 2 e- Pb (s)
Fe2+ (aq) + 2 e- Fe (s)
E(Cu2+/Cu) = 0.337 V
E(Zn2+/Zn) = -0.763 V
E(Pb2+/Pb) = -0.13 V
E(-Fe2+/Fe) = -0.44 V
Fe has a thermodynamic tendency to
reduce Pb2+ (aq)
Thermodynamic Information
Note
G
FE r G
T ,P
And
FE r G
Entropy Changes
To obtain the entropy change for the
cell reaction
S
rxn S
T
T ,P
rxn S
rxn G
T
G
T ,P
T ,P
F E
T P
E
F
T P
Enthalpy Changes
To obtain the enthalpy change for the cell reaction
H
rxn H
G
T ,P
T ,P
E
FE FT
T
rxn H FE
S
T
P
E
FT
T
P
T ,P
The Liquid Junction Potential
Examine the following electrochemical
cell
Activity difference of the HCl between
compartment 1 and compartment 2
There should be a transport of matter
from one cell compartment to the
other!
A Concentration Cell
e-
0.0592V
e-
Porous Disk or
Salt Bridge
Ag(s)
a(Cl-) = 0.010
Left
a (Cl -) = 0.0010
Right
Ag(s)
The Development of Liquid Junction
Potentials
The cell
compartments
are identical
except for the
activities of
the electrolyte
solutions.
HCl (a2)
HCl (a1)
Ag/AgCl electrode
Note that we
now have the
migration of both
cations and
anions through
the liquid
junction.
H+
Cl-
Ag/AgCl electrode
After a
period of
time
+++++
- - - - -----------
Ag/AgCl electrode
Choose the lower compartment as our
LHS electrode.
Ag AgCl
Cl- (aq) a1 Cl- (aq), a2 AgCl (s) Ag (s)
Note: liquid junction
For
the passage of one mole of charge
through the cell
-F Ecell = GJ
The Cell Reactions
For the LHS and RHS electrodes
AgCl (s) + e- Ag (s) + Cl- (a1) LHS
AgCl (s) + e- Ag (s) + Cl- (a2) RHS
Net change
Cl- (a1) Cl- (a2)
Note that the charge at the interface is
transported by the anions and cations
in the cell reaction!
The Transport Numbers
How is the charge carried at the interface
of the cells?
t+ moles of charge carried by the H+
(cation).
t- moles of charge carried by the Cl(anion).
Passage of one mole of “+” charge through
the interface
requires the passage of t+ moles of H+ (aq) from
the LHS RHS, and the passage of t- mole of Clcharge from the RHS LHS.
At the boundary
t+ H+(a1) + t- Cl-(a2) t+ H+(a2) + tCl-(a1)
For the entire cell
Cl- (a1) t+ H+(a1) + t- Cl-(a2) Cl- (a2)
t+ H+(a2) +
t- Cl-(a1)
The cell reaction involves the
transport of t+ moles of HCl from the
LHS to the RHs of the cell.
The Gibbs Energy Changes
For the above cell reaction, we can
write the Gibbs energy expressions as
follows
G t (H ) RT lna (H )2 (Cl ) RT lna (Cl )2
t (H ) RT ln a (H )1 (Cl ) RT ln a (Cl )1
G t
a (H
RT ln
a (H
)a (Cl ) 2
)a (Cl ) 1
Cells With Transference
Note a(H+) a (Cl-) = {a (HCl)}2
G
a (HCl )2
2 t RT ln
a (HCl )1
Note that the cell potential with
transference, Ewt is determined as follows
E wt
RT a (HCl )2
2 t
ln
a (HCl )1
F
Cells without Transference
What if we were able to set up a cell so that
the transport at the interface did not
contribute to the overall G?
The potential of this cell would be the cell
potential without transference, Ewot.
Cl- (a1) Cl- (a2)
G RT
E wot
a (HCl )2
ln
a (HCl )1
RT a (HCl )2
ln
a (HCl )1
F
The Liquid Junction Potential
The liquid junction potential is the
difference in the cell potentials with
and without transference!
E LJ E wt E wot
E LJ
RT a (HCl )2
1 2 t
ln
a (HCl )1
F
L.J. Potentials Depend on
Transport Numbers
What is the following were true?
t+ t- 0.5 ELJ would be very small
and would only make a small
contribution to the overall cell
potential !
L.J. Potentials Depend on
Transport Numbers
ELJ a potential problem any time we
measure the cell potential whose
electrodes have different electrolytes
How does the salt bridge help?
e.g., for species with t+ t- 0.5, the ELJ
values are small and are readily
established!