Experiment: Determination of a Ksp
advertisement

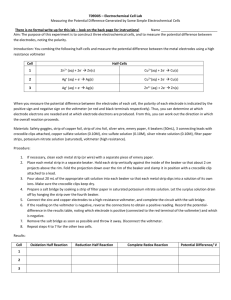
CHEM 225 LAB #7 – Building Electrochemical Cells Determining the Potential of Electrochemical Cells OBJECTIVE: In this lab experiment, you will build several electrochemical cells and then determine the potential o In an electrochemical cell, chemical energy is converted into electrical energy. This is accomplished by using a spontaneous chemical reaction to generate an electric current, which we can simply define here as electrons traveling though a wire. THEORY: To create the electrochemical cell, two half-reactions will be set up in different containers. In one, an oxidation reaction will be used to generate a source of electrons which will then travel, through an external circuit, to a second container where reduction takes place. A salt bridge will connect the two half cells to allow ions to flow for preventing charge buildup. EXPERIMENTAL PROCEDURE – you will work in groups for this lab 1. Make sure that you know how to use the DC voltmeter that is at your lab station. 2. General Information for each electrochemical cell that you will make is below. a. Create each half-cell by placing a metal electrode in an electrolytic solution containing the same metal’s ions. For example the copper electrode will be placed in a copper(II) nitrate solution (already made for you). b. Each electrochemical cell you create you will require two half-cells. Set these cells (beakers) beside each other – they will be connected by a U-tube (glass tube which you will fill with KNO3). c. Fill each beaker about two-thirds full of the electrolytic solution. Clean the electrode using the steel wool, then place the electrode in its appropriate solution. d. Clip one end of each copper wire to the two electrodes using the alligator clips. e. Fill the U-tube with KNO3 and stopper both ends with the cotton plugs. Turn the Utube upside down and place one end in each half-cell. f. Touch the other end of the copper wires to the voltmeter terminals. If the indicator on the voltmeter deflects in the wrong direction, switch the wires on the terminals. Read the highest voltage reading obtained – you’ll need to do this quickly after connecting the wires to the voltmeter. DATA AND CALCULATIONS 1. All of the solutions are nitrate solutions of the cation. For example, for Cu2+, you have a Cu(NO3)2 solution. 2. Determine the cell potential (Voltage) of the following combinations of half-cells using your electrochemical setup (directions above) and your voltmeter. Half-cells Cu|Cu2+ Zn|Zn2+ Cu|Cu2+ Pb|Pb2+ Zn|Zn2+ Pb|Pb2+ Cell Voltage 3. Compare the cell potentials your determined today with the theoretical potentials that you pre-determined from the Standard Reduction Potential. 4. From your date above, using the Nearnst equation, deduce the concentration of Cu2+ in the electrolyte solution that was made for you.