DiffusionLab
advertisement

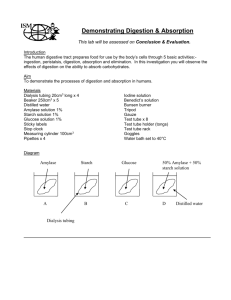
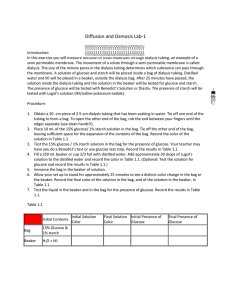
IB Biology II Laboratory 1: Diffusion Objective: In this laboratory, you will study some of the basic principles of molecular movement in solution and perform a series of activities to investigate these processes. Background: Molecules are in constant motion and tend to move from regions where they are in higher concentration to regions where they are less concentrated. Diffusion is the net movement of molecules down their concentration gradient. Diffusion can occur in gases, in liquids, or through solids. There are often several different types of molecules in a solution. The motion of each type of molecule is random and independent of other molecules in the solution. Each molecule moves down its own concentration gradient, from a region of its high concentration to a region of its low concentration. Though the net movement of molecules is down their concentration gradient, at any time molecules can move in both directions as long as the membrane is permeable to the molecule. Since the cell membrane is a selectively permeable membrane, that is, it lets some substances in and out of the cell while not allowing other substances to pass at all. Molecules that are large, such as proteins and starch, and charged particles, such as ions (NA+, K+) usually are not able to get through the cell membrane unless the appropriate protein “gate” is present. Materials: 50 mL beaker 2 - 250 mL beaker 15 cm soaked dialysis tubing 15 mL 15% glucose/1% starch solution 1 pipet 1 mL stach indicator solution (IKI) 2 - 10 cm pieces of string Distilled H20 1 mL Benedict’s solution Test tube Hot plate Tap water Timer Procedure: 1. Put on your goggles. 2. Fill the 250 mL beaker with 125 mL of distilled water. 3. Carefully add 10 mL of the starch indicator solution (IKI) to the distilled water. Observe and record what happens to the indicator solution as it mixes with the water. 4. Get a soaking segment of dialysis tubing. Gently rub the tubing between your fingers to open it. 5. Tie one end of the tubing tightly with a piece of string. Make sure this end is tied tightly enough to prevent any leaks from the end of the bag. Fill the tubing with tap water and test it for leaks at the sink. Empty the tubing. 6. Carefully pour your 15 mL of 15% glucose/1% starch solution into your dialysis tubing. 7. Squeeze all the air bubbles out of the tubing and tie the open end shut with another piece of string. Note the color of the glucose/starch solution in the dialysis tubing. 8. Rinse the outside of the bag under running water. Squeeze the bag gently to ensure there are no leaks. 9. Completely submerge the dialysis tubing (which is now a model of a cell) into the cup of distilled water and IKI. Allow osmosis and diffusion to occur for 30 minutes. 10. After 30 minutes, test the water in the beaker for the presence of sugar content. You can do this in two ways since we need to test for the presence of glucose and starch. a. Do this first: To test for the presence of glucose: Put a small amount (~ 5mL) of your distilled water/IKI solution into a test tube with a small amount (~ 1 mL) of Benedict’s solution. Put your test tube into a hot water bath for approximately 510 minutes; during a water bath, the solution should progress in the colors of blue (with no glucose present), green, yellow, orange, red, and then brick red or brown (with high glucose present). Record your results. b. Do this second: To test for the presence of starch: If starch diffused out of the dialysis tubing and into the beaker, then the starch indicator solution (IKI) would turn a blue/black color. If starch did not diffuse out of the dialysis tubing and into the beaker, then the IKI would turn an orange/brown color. Record your results. Then, open your dialysis bag in the beaker; note any changes before and after you open it up. 11. Clean up your lab station and return all materials back to where you found them. For this lab write-up, you will need to complete the Conclusion & Evaluation as well as the Abstract section. At least one outside source will need to be used and cited in your Conclusion & Evaluation. This section is due on _______________________________.