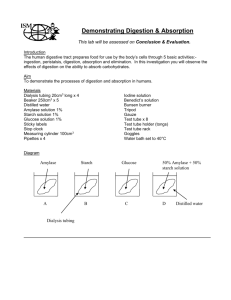
Figure 1 shows the possible solutes for part A of the lab. As you can
advertisement

Figure 1 shows the possible solutes for part A of the lab. As you can see solutes have various shapes, sizes and properties. The selective permeability of the membrane much like a filter. is Part A Introduction: In part A you and a partner will be exploring the selective permeability of a membrane, dialysis tubing. Dialysis tubing is much like a cell membrane in that it only lets certain molecules through. We will be concentrating on Starch and Glucose. Remember that there is a size difference between the two molecules with starch being a polymer and glucose being a monomer. You will be using two indicators to test for both substances. IKI solution reacts with starch and bonds to starch. It does what starch does or it moves with starch. You will notice a dark blue color to the solution when there is starch present. Glucose will be tested using test strips. The shade of brown indicates the presence and concentration. Materials: Dialysis tubing, plastic cup, glucose/starch solution, distilled water, IKI solution, dropper, glucose test strips, funnel and string. Procedure: 1. Pour water into a beaker about 2/3 full. Then add about 4 ml of IKI solution to the cup and mix until fully dispersed. Then test the water/IKI solution with a glucose strip. Record your data for both glucose and starch in your data table. Use a (+) for a positive test and a (-) for a negative test. 2. Test the glucose/starch solution for glucose. 3. Tie one end of the soaked dialysis tubing and use a funnel to add 15 ml of the glucose/starch solution into the bag. Leave some room for expansion and tie the other end. Record the initial color of the solution in the tubing. 4. Fully immerse the tubing into the water/IKI solution for 30 minutes. 5. While waiting complete the following. 1. Label the location of all possible solutes in this activity. Assign a different symbol for different solutes. Include IKI. 2. In the space provided predict the movement of all your labeled solutes. Prediction 1: ____________________________________________ ____________________________________________ ____________________________________________ ____________________________________________ Prediction 2: ____________________________________________ ____________________________________________ ____________________________________________ ____________________________________________ Prediction 3 ____________________________________________ ____________________________________________ ____________________________________________ ____________________________________________ Analysis: 1. Explain your results in a short paragraph conclusion. 2. How did your results compare to your predictions? 3. Does the activity account for all the possible solute movement? If not, how could we account for all of them? 4. What does your data tell you about the size of the solutes in comparison to the permeability of the tubing? Part B Osmosis Introduction: In part B you will investigate the influence on solute concentration on the movement of water across a membrane. (osmosis) The solute you will use is Sucrose (table sugar – disaccharide). You will use varying concentrations starting at 1 M going down to 0 M. You will be splitting the work up as a class making sure all the concentrations are accounted for. Solutions will be premixed by Mr. D. Materials: Dialysis tubing, string, water, plastic cups, funnel, sucrose solutions, paper towels, balance, calculator. Procedure: 1. Fill a labeled (See data table) 2/3rds full of water. 2. Tie a string at one end of soaked dialysis tubing and pour 25 ml of the varying sucrose solutions in the tubing and close the top with string. Leave room for expansion and repeat for all tested solutions concentrations. 3. Dry bag with paper towel and weigh. Record initial mass on table and fully submerge tubing in corresponding labeled cups. Let stand for 30 minutes. Record a hypothesis below Hypothesis: ________________________________________________________________________________________________ ______________________________________________________________________________________ 4. After 30 minutes, take tubing out, dry and weigh. Record final mass on table. 5. Calculate % change and answer conclusion questions. Conclusion Questions: 1. What does the change in mass indicate? 2. What variable is being tested in this experiment 3. Identify at least three other variables that could influence the results of this activity. Include how you controlled them or could control them better. 4. Graph the percent changes. First identify: A. Independent Variable _____________________________ B. Dependent Variable ______________________________ 5. Do your results support your hypothesis, refute it, or require that you modify the experiment. 6. Based on your results write a conclusion statement that sums up the relationship of solute concentrations and the net movement of water across a semi-permeable membrane. 7. Which trial was an isotonic situation? 8. What would happen if water was in the tubing and the varying solute concentrations were in the cups? 9. How can drinking seawater actually dehydrate a body?