Cooking With Vegetable Oils
advertisement

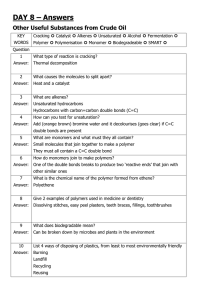
Finishing off from last time Fatty Acids The long fatty acid chains stop vegetable oils dissolving in water The fatty acids in some vegetable oils are saturated, and only have single bonds between their carbon atoms Saturated oils tend to be solid at room temperature, and are sometimes called vegetable fats instead of oils Lard is an example of a saturated oil Fatty Acids The fatty acids in some vegetable oils are unsaturated, and have double bonds between some of their carbon atoms Unsaturated oils tend to be liquid at room temperature, and are useful for frying food Unsaturation The carbon-carbon double bonds in unsaturated oils can be detected using the elements bromine or iodine – these elements react with the double bonds in the oils, and the more double bonds there are, the more bromine or iodine is used up Unsaturated fats can be tested for using a simple test with bromine water – bromine water is a dilute solution of bromine, which is normally orangebrown in colour which becomes colourless when shaken with an alkene, or with unsaturated fats When shaken with alkanes or saturated fats, its colour remains the same Unsaturation Quick Practical: Use Bromine water to test for saturation of the products on the side Thursday, March 24, 2016 To know how plant oils are useful in cooking The fatty acids in some vegetable oils are saturated – how many bonds do they have between their carbon atoms? Saturated oils have single bonds between their carbon atoms – they tend to be solid at room temperature (e.g. lard) The fatty acids in some vegetable oils are unsaturated – how many bonds do they have between their carbon atoms? Unsaturated oils have double bonds between some of their carbon atoms – they tend to be liquid at room temperature and can be divided into two categories: • Monounsaturated fats have one double bond in each fatty acid • Polyunsaturated fats have many double bonds During hydrogenation, vegetable oils are hardened by reacting them with hydrogen gas at about 60ºC (this increases their melting point) A nickel catalyst is used to speed up the reaction – the double bonds are converted to single bonds by the hydrogenation This causes unsaturated fats to be made into saturated fats… Saturated vegetable oils are solid at room temperature, and have a higher melting point than unsaturated oils This makes them suitable for making margarine, or for commercial use in the making of cakes and pastry Complete the cooking in oil and water experiment The temperature that a liquid boils at depends on the size of the forces between its molecules – the bigger these forces the higher the liquid’s boiling point The molecules in vegetable oils are much bigger than water molecules (so their boiling point is much higher) Cooking food causes permanent changes to occur to the food – cooking in vegetable oils causes different reactions to the food as the temperature is so much higher (often the food cooks more quickly, turns a different colour on the outside and becomes crisper) Also cooking in oil can cause the food to absorb some of that oil meaning the energy content of the food is much higher (one reason why fried food can be bad for you)! Vegetable oils are important nutrients and provide a lot of energy. Vegetable oils are also used as fuels for vehicles (some of this biodiesel is made from waste cooking oil and rapeseed oil with benefits as these fuels are carbon neutral) It can be questioned how ethical it is to use food crops in this way, instead of using them for feed when famine is still a global problem… Vegetable oils do not dissolve in water – if a mixture of oil and water is shaken, then left to stand, eventually a layer of oil will form on the surface of the water Emulsifiers can be added to the oil and water, causing an emulsion to form (a mixture of the two) Emulsions are more viscous than oil or water on their own, and contain tiny droplets of one of the liquids spread through the other liquid Emulsifier molecule – hydrophilic head and hydroscopic tail Water Oil droplet Examples of oil droplets in water: • Egg yolk • Milk • Ice cream • Salad cream • Mayonnaise Examples of water droplets in oil: • Margarine • Butter • Skin cream • Moisturising lotion Partially hydrogenated vegetable oils may contain trans fats These are thought to cause health problems such as heart disease in humans, and food manufacturers are being encouraged to reduce the amount of them in our food…