Explorations of Oils and Fats
advertisement

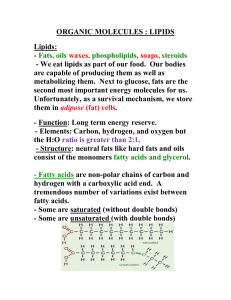
Fats and Oils Explorations Background: Fats, oils, waxes, cholesterol, steroid hormones and Vitamins A and D – all of these natural products belong to the diverse class of biological compounds called lipids. The biological functions of lipids are as diverse as their structures. Fats and oils, for example, are used to store energy within cells and organisms. In addition to acting as energy sources, fats accumulate in adipose tissue that insulates and protects internal organs. Phospholipids are responsible for the “lipid bilayer” structures that form protective membranes around cells. The steroid hormones, which are synthesized in the body from cholesterol, act as chemical messengers, carrying signals from one part of the body to another. Fats and oils – referred to collectively as triglycerides – have the same basic structure. Triglycerides consist of a glycerol backbone and three attached fatty acid residues. Fatty acids are long-chain carboxylic acids, consisting of a long hydrocarbon “tail” with a carboxyl group at one end. Fatty acids range in length from 10 to 20 carbon atoms and always contain an even number of carbon atoms. The hydrocarbon chains in fatty acids can be saturated with hydrogen atoms or unsaturated (monounsaturated or polyunsaturated). Fats are solids and are primarily obtained from animal tissue. Oils are liquids and are primarily obtained from plants. Olive oil is an example of an oil, while lard is an example of a fat. glycerol + 3 fatty acids triglyceride Saturated fatty acid Monounsaturated fatty acid Polyunsaturated fatty acid Purpose: To determine the properties of fats and oils. Materials: Olive oil, canola oil, lard, coconut oil, water, acetone, sudan III stain, ethanol, crucible, burner, brown paper, dish soap, Petri dish Safety: Wear gloves when handling the sudan III stain. Procedure: 1. Place some oil into the crucible and heat from underneath with a butane burner. 2. On separate spots of the brown paper, place a drop of oil, a smear of lard, and a drop of water. 3. Place 3 drops of oil onto the Petri dish in three separate locations. Add 1 drop of water to one of these oil drops. Add 1 drop of ethanol to another one of these oil drops. Add 1 drop of acetone to the third oil drop. Stir each drop combination with a designated toothpick. Record your observations. 4. Repeat step 3 with a small amount of lard. 5. Use a clean Petri dish. Place a drop of water in one location, a drop of acetone in another location, a drop of oil in a third location, and a dab of lard in a fourth location. Add a tiny bit of sudan III stain to each of these substances and stir with a designated toothpick. Record your observations. 6. Fill half of a test tube with water. Use the pipette to add oil to the bottom of the test tube. Record observations. 7. Cover and shake the test tube and record your observations. 8. Add a couple drops of dish soap to the test tube and repeat step 7.