Lecture 13: Cell Potentials
advertisement

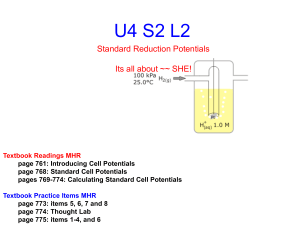
Lecture 11: Cell Potentials • Reading: Zumdahl 11.2 • Outline – What is a cell potential? – SHE, the electrochemical zero. – Using standard reduction potentials. Reminder • “Redox” Chemistry: Reduction and Oxidation • Oxidation: Loss of electrons (increase in oxidation number) • Reduction: Gain of electrons (a reduction in oxidation number) • Electrons are transferred from the reducing agent to the oxidizing agent • Electrons are transferred from the species being oxidized to that being reduced. Galvanic Cells (cont.) 8H+ + MnO4- + 5eFe2+ Mn2+ + 4H2O Fe3+ + e- x5 Galvanic Cells (cont.) • Galvanic Cell: Electrochemical cell in which chemical reactions are used to create spontaneous current (electron) flow Galvanic Cells (cont.) Anode: Electrons are lost Cathode: Electrons are gained Oxidation Reduction Cell Potentials • In a galvanic cell, we had a species being oxidized at the anode, a species being reduced at the cathode, and electrons flowing from anode to cathode. • The force on the electrons causing them to full is referred to as the electromotive force (EMF). The unit used to quantify this force is the volt (V) • 1 volt = 1 Joule/Coulomb of charge V = J/C Cell potential and work • From the definition of electromotive force (emf): Volt = work (J)/charge (C) • 1 J of work is done when 1 C of charge is transferred between a potential difference of 1 V. Cell Potentials (cont.) • We can measure the magnitude of the EMF causing electron (i.e., current) flow by measuring the voltage. e- Anode Cathode 1/2 Cell Potentials • What we seek is a way to predict what the voltage will be between two 1/2 cells without having to measure every possible combination. • To accomplish this, what we need to is to know what the inherent potential for each 1/2 cell is. • The above statement requires that we have a reference to use in comparing 1/2 cells. That reference is the standard hydrogen electrode (SHE) 1/2 Cell Potentials (cont.) • Consider the following galvanic cell • Electrons are spontaneously flowing from the Zn/Zn+2 half cell (anode) to the H2/H+ half cell (cathode) 1/2 Cell Potentials (cont.) • We define the 1/2 cell potential of the hydrogen 1/2 cell as zero. SHE P(H2) = 1 atm [H+] = 1 M 2H+ + 2e- H2 E°1/2(SHE) = 0 V 1/2 Cell Potentials (cont.) • With our “zero” we can then measure the voltages of other 1/2 cells. • In our example, Zn/Zn+2 is the anode: oxidation Zn+2 + 2e- Zn 2H+ + 2e- Zn + 2H+ H2 E°Zn/Zn+2 = 0.76 V E° SHE = 0 V Zn+2 + H2 E°cell = E°SHE + E°Zn/Zn+2 = 0.76 V 0 Standard Reduction Potentials • Standard Reduction Potentials: The 1/2 cell potentials that are determined by reference to the SHE. • These potentials are always defined with respect to reduction. Zn+2 + 2e- Zn E° = -0.76 V Cu+2 + 2e- Cu E° = +0.34 V Fe+3 + e- Fe+2 E° = 0.77 V Standard Potentials (cont.) • If in constructing an electrochemical cell, you need to write the reaction as a oxidation instead of a reduction, the sign of the 1/2 cell potential changes. Zn+2 + 2eZn Zn E° = -0.76 V Zn+2 + 2e- E° = +0.76 V • 1/2 cell potentials are intensive variables. As such, you do NOT multiply them by any coefficients when balancing reactions. Writing Galvanic Cells For galvanic cells, Ecell > 0 In this example: Zn/Zn+2 is the anode Zn+2 + 2e- E° = +0.76 V Zn Cu/Cu+2 is the cathode Cu+2 + 2e- Cu E° = 0.34 V Writing Galvanic Cells (cont.) Zn+2 + 2e- E° = +0.76 V Zn Cu+2 + 2e- Cu E° = 0.34 V Cu+2 + Zn Cu + Zn+2 E°cell = 1.10 V Notice, we “reverse” the potential for the anode. E°cell = E°cathode - E°anode Writing Galvanic Cells (cont.) Shorthand Notation Zn|Zn+2||Cu+2|Cu Anode Cathode Salt bridge Predicting Galvanic Cells • Given two 1/2 cell reactions, how can one construct a galvanic cell? • Need to compare the reduction potentials of the two half cells. • Turn the reaction for the weaker reduction (smaller E°1/2) and turn it into an oxidation. This reaction will be the anode, the other the cathode. Predicting Galvanic Cells (cont.) • Example. Describe a galvanic cell based on the following: Ag+ + e- Ag E°1/2 = 0.80 V Fe+3 + e- Fe+2 E°1/2 = 0.77 V Weaker reducing agent – turn it around Ag+ + Fe+2 Ag + Fe+3 E°cell = 0.03 V E°cell > 0….cell is galvanic Another Example • For the following reaction, identify the two half cells, and use these half cells to construct a galvanic cell 3Fe+2(aq) +2 Fe(s) + 2Fe+3(aq) reduction 0 +3 oxidation Fe+2(aq) + 2e- Fe(s) E° = -0.44 V Fe+3(aq) + e- Fe+2(aq) E° = +0.77 V Another Example (cont.) weaker reduction – turn it around Fe+2(aq) + 2e2 x Fe+3(aq) + e- 2Fe+3(aq) + Fe(s) Fe(s) Fe(s) E° = -0.44 V Fe+2(aq) E° = +0.77 V 3Fe+2(aq) Fe+2(aq) + 2e- E°cell = 1.21 V E° = +0.44 V