Chem of Life Notes
advertisement

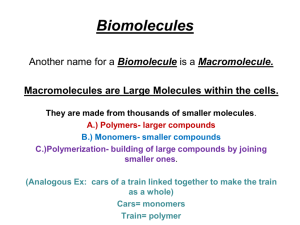
Basic Chemistry of Life Matter • • • • • anything that occupies space and has mass Composes elements C, H, O, and N make up 96% of human mass. C, H, O, N, P, and Ca make up 99% of human mass. Can undergo physical and chemical changes All living things are built of matter and require energy to grow and function. Mass • the measure of the amt of matter Elements and Atoms • Atomic Structure: Electrons: - charge Protons : + charge Neutron: no charge Surround the nucleus like a cloud Nucleus of an atom • Atomic # = # of protons • # protons = # of electrons • Atomic mass = # of protons + # of neutrons • Atomic weight = # of protons and # neutrons (not an even # b/c It is the avg of the isotopes) ATOMS Compounds vs Ions • Compounds -two or more elements IONS - ex: CO2, H2O, C6H12O6 • Ions -electrically chgd atoms -Give or take e-’s from other atoms -(+) lose e- = CATION, (-) gain e- = ANION Chemical Bonding • Covalent bonds - shared electrons - ex: H2O, CO2 • Ionic bonds -transfer electrons -ex: NaCl • Hydrogen bonds -weak chem bonds to either N or O atoms in proteins and Acids, bases, and the pH scale Acids are molecules that can give up H+ ions Bases are molecules that can accept H+ ions pH is a measurement of the relative concentration of H+ ions as compared to water • Adding an acid to water will increase H+ concentration making the solution acidic • Adding a base to water will decrease the H+ concentration making it basic • pH scale ranges from 0 (very acidic) to 14 (very basic) -----Water pH = 7.0 Catabolic and Anabolic reactions •In living cells, molecules are constantly being broken down into smaller molecules (catabolic reaction) or built up into larger molecules (anabolic reactions) •Anabolic reactions often USE energy, catabolic reactions often RELEASE energy Example: The breakdown of ATP into ADP + P yields energy ATP + H2O anabolic ADP + P + ENERGY (to be used by cell) catabolic “What ANA makes, the CAT breaks” Chemical Rxns • Synthesis (A + B AB) -ex: 2H2 + O2 2H2O -forming compds…..growth and repair of body tissues • Decomposition (AB A + B) -ex: 2H2O 2H2 + O2 -break down of food and destruction of cells • Exchange (AB + CD AD + CB) -ex: NaOH + HCl NaCl + H2O -blood using carbonic acid as a buffer • Inorganic compounds -lack carbon compds -ex: 1. water -most metabolic rxns -transports chemicals -absorbs and releases heat, lubricates and protects 2. oxygen -respiration -required to release energy from sugars 3. CO2 -waste product of cell respiration 4. acids, bases and salts -nerve impulses and muscle contractions -acids release H+ ions in water, bases release OH- ions -buffers eliminate extreme fluctuations in pH (H2CO3) Acid + Base Salt HCl + NaOH NaCl (salt) + H2O • Organic Compounds -contain carbon and usually hydrogen -covalently bonded -ex: carbohydrates, lipids, proteins and nucleic acids Formation and Breakdown of Organic Molecules -built by bonding small molecules (monomers) to form long chains called polymers. • Dehydration synthesis – remove H2O to form bonds -ex: protein synthesis • Hydrolysis – add H2O to break bonds -ex: digestion Carbohydrates • Sugars and starches • C, H, O (1C: 2H ratio) • Store and release energy 3 Types 1. Monosaccharides – single sugars -glucose, galactose, fructose, deoxyribose, ribose 2. Disaccharides – double sugars -sucrose, maltose, lactose 3. Polysaccharides – many sugars -cellulose, starch, glycogen, chitin Carbohydrate Monomers “Monosaccharides” 3 Primary carbohydrate monomers 1. Glucose-main energy source for most cells, brain cells and nerve cells actually demand it • Commonly referred to as “blood sugar” 2. Galactose-chemically similar to glucose • Body converts galactose to a glucose derivative for use 3. Fructose-common sugar found in fruits • Also converted to a glucose derivative before use Disaccharides 3 Primary Disaccharides 1. Sucrose-common table sugar, cane sugar Digested to 1 glucose and 1 fructose 2. Lactose-milk sugar Digested to 1 glucose and 1 galactose 3. Maltose-malt sugar that comes from starch digestion Digested to 2 molecules of glucose Disaccharides are linked via DEHYDRATION SYNTHESIS reactions and broken down into monosaccharides by HYDROLYSIS All the disaccharides are metabolized to glucose and some “other” sugar • The “other” sugar is also eventually converted to glucose as well • Obviously, glucose is a very important energy source! Polysaccharides •Large carbohydrate polymers made up of long chains of GLUCOSE monomers 1. Cellulose - Structural carbohydrate in plants Can’t be digested by humans Also referred to as “dietary fiber” or roughage like lettuce 2. Starch - Energy storage carbohydrate in plants The main digestible polysaccharide in our diets Potatoes, rice and wheat have a lot of starch 3. Glycogen – Energy storage carbohydrate in HUMANS Readily accessible source of energy stored up when we eat excess food Primarily stored in liver, but also found in muscle and brain tissues Lipids • • • Fats, phospholipids and steroids C, H, O but no ratio (C57H110O8) Protect, insulate and provide energy storage 3 Types 1. Triglycerides -3 fatty acids and glycerol 2. Phospholipids -2 fatty acids, glycerol and phosphate 3. Steroids -4 rings (cholesterol) -HDL (good cholesterol), LDL (bad cholesterol) Lipids 1. Saturated – only single carbon to carbon bonds -solid at room temp -ex: butter, lard, steak fat 2. Unsaturated – contain some double carbon to carbon bonds -liquid at room temp -ex: olive oil, canola oil, peanut oil Proteins • 50% of the organic matter in the body • C, H, O, N and sometimes S • Composed of monomers known as Amino acids (20 different) Uses 1. Make up cell membranes 2. Transport 3. Build structures (like muscles and tendons) 4. Carry out metabolic processes (enzymes) 5. Defensive of the body (antibodies) Enzymes • Protein catalysts -substrate specific (lock and key / induced fit) Substrate – the reactant that an enzyme works upon Active site – where the rxn takes place on the enzyme Factors that affect enzymes -pH, temp, [enzyme], [substrate], salt Changes in temp, pH and [salt] can cause enzymes to denature Nucleic Acids • Composed of nucleotides -Sugar, Phosphate, Nitrogen base -DNA and RNA DNA- double helix RNA – single helix Construct proteins, enzymes, metabolic process ATP – Adenosine Triphosphate • Energy sourced used in all life processes • Composed of Adenine, ribose sugar and 3 phosphates ATPADP + P (release energy) ADP + P ATP (store energy)