Electrons In Atoms
advertisement

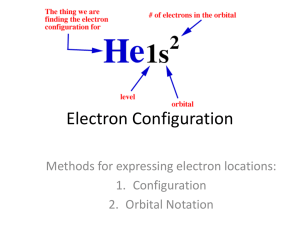
Electrons In Atoms Electromagnetic Radiation • Form of energy that exhibits both wavelike behaviors and particle behaviors Electromagnetic Spectrum • Shows all forms of electromagnetic radiation Quanta • Electrons gain/lose energy in set increments only • Each set amount of energy is called a quantum • Lost energy is released as electromagnetic radiation, with more energy = higher frequency Photoelectric Effect • Emission of electrons from metal’s surface when light of specific frequency shines on surface light eMETAL Wrap-Up #1 • If electrons will only be emitted by light of high energy, which of the following is more likely to release electrons? – Blue light with low intensity (dim) – Red light with high intensity (bright) – Radio waves with high intensity Bohr Model – Electron States • Ground State – Lowest energy state of an electron – Where the electron is “naturally” • Excited State – State when an electron gains energy – Only exists while energy is being absorbed by the atom • Glow in the dark materials – the electrons absorb energy from light and re-release it as light when its surroundings are dark Bohr Model E1 = lowest energy level E1 E3 > E2 > E1 E2 E3 Ground State to Excited State 4 Energy of atom 1. in ground state, no energy radiated 2. in excited state, electrons jump to higher energy level (because they’ve absorbed energy from an external source) 3. electrons go from high E level to low E level 4. photon emitted 6 5 4 3 3 2 2 1 1 Atomic Orbitals • Volume surrounding the nucleus in which an electron is 90% likely to be found Principle Quantum Number (n) • Indicates the energy level an electron is on – Use periodic table to tell – The period number corresponds to the principle quantum number (n = 1,2,3…) Energy Orbitals • Shape of orbital that tells the path of the electrons – 4 orbitals: s, p, d, f – The letter tells you the shape of the orbital s orbital • Shape: electrons travel in a sphere s orbital 3s 1s 2s The greater the energy level, the bigger the orbital p orbital • Shape: dumbbell or figure 8 shaped d orbital • Shape – double dumbbells or a dumbell with a ring around it Electron Configuration • Description of the arrangement of electrons in an atom • Allows us to visualize where the electrons in an atom can be found Rules Governing Electron Configurations 1) Aufbau Principle – electrons occupy lowest energy orbital available - fill up level 1 first, then level 2, etc. 2) Pauli Exclusion Principle – there is a max number of electrons that occupy a single orbital (2) and they must have opposite spin Rules Governing Electron Configurations 3) Hund’s Rule – if orbitals have equal energy, one e- will go in each orbital before doubling up 1 2 3 4 5 6 Rules Governing Electron Configurations 3b) Hund’s Rule – all electrons in singly occupied orbitals must have the same spin Yes Yes Yes NO NO NO Blocks On Periodic Table s s p d f Divisions of Orbitals • s orbital – 1 sublevel (2 e- max) • p orbital – 3 sublevels (6 e- max) • d orbital – 5 sublevels (10 e- max) • f orbital – 7 sublevels (14 e- max) Orbital Diagram • Nitrogen • How many electrons? 7 1s 2s 2p Orbital Diagram • Silicon • How many electrons? 14 1s 2s 2p 3s 3p Wrap-Up #2: Orbital Diagram • Copper • How many electrons? 29 1s 2s 4s 2p 3d 3s 3p Electron Configuration Notation • Oxygen (8 e-) 2 2 1s 2s 2p 4 • Sulfur (16 e-) 2 2 6 2 1s 2s 2p 3s 3p 4 • Vanadium (23 e-) 2 2 6 2 6 2 1s 2s 2p 3s 3p 4s 3d 3 Noble Gas Notation • Rule: start from previous noble gas, then write the configuration • Oxygen [He] 2s 2 2p 4 2 4 [Ne] • Sulfur 3s 3p 2 3 [Ar] 4s 3d • Vanadium Valence Electrons • Electrons in outer most energy level - located in highest s & p orbitals N: 2 2 3 5 valence e 1s 2s 2p Mg: 2 2 6 1s 2s 2p 3s 2 2 valence e- Se: 1s2 2s2 2p 6 3s2 3p6 4s 2 3d10 4p 4 6 valence e- Electron Dot Structure • Shows valence electrons in a diagram • Nitrogen (5 v.e.) N • Magnesium (2 v.e.) Mg • Selenium (6 v.e.) Se