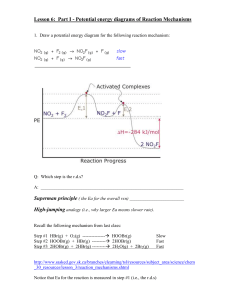
Assistance Lecturer Amjad Ahmed Jumaa Standard enthalpy of
advertisement
Inorganic chemistry
Standard enthalpy of formation and reaction.
Calculating the standard enthalpy of reaction
Direct method of calculating the standard
enthalpy of formation.
Assistance Lecturer Amjad Ahmed Jumaa
www.soran.edu.iq
1
Standard enthalpy of formation and reaction:
The standard enthalpy of formation (ΔHf° ) is defined as the heat
change that results when one mole of a compound is formed from its
elements at a pressure of (1 atm
Calculating the standard enthalpy of reaction
From standard enthalpies of formation, we can calculate the
standard enthalpy of reaction (ΔH°rxn). Consider the following
hypothetical reaction.
www.soran.edu.iq
aA + bB → cC + dD
Where, a,b,c and d are stiochiometric coefficients.
The standard enthalpy of reaction is given by:
ΔH° rxn = [ c ΔH°f ( C) + d ΔH°f (D) ] – [ a ΔH°f (A) + b ΔH°f (B)]
Where a,b,c and d all have the unit mol.
The equation can be written in the general form:
ΔH° rxn = ( (Σn ΔH°f (products) –( Σm ΔH°f (reactnts) )
Where (m) and (n) denote the stiochiometric coefficients for
the reactants and products ,and ( Σ ) (sigma) means '' the sum
of''.
www.soran.edu.iq
Example:
A reaction used for rocket engines is
N2H4 (l) + 2H2O2 (l) → N2 (g) +4H2O (l)
What is the standard enthalpy of reaction in kilojoules ? the
standard enthalpies of formation are:
ΔH°f [ N2H4 (l)] = 95.1 kJ.
ΔH°f [ H2O2(l)] = - 187.8 kJ.
ΔH°f [ H2O(l) ] = - 285.8 kJ.
Solution :
ΔH°rxn = ( (Σn ΔH°f (products) –( Σm ΔH°f (reactnts) )
ΔH°rxn = ΔH°f [N2 (g) ] +4ΔH°f [H2O(l)]-{ ΔH°f [N2H4(l)]+2[H2O2(l)]}
www.soran.edu.iq
Remember , the standard enthalpy of formation of any element
in its most stable form is zero. Therefore,
ΔH°f [ N2(g)] = 0.
ΔH°rxn= [ 0 + 4(-285.8 kJ)] –[ 95.1 kJ+ 2(- 187.8 kJ)] = 862.7 kJ.
Direct method of calculating the standard enthalpy of
formation
This method of measuring (ΔH°f) applies to compounds that
can be readily synthesized from their elements. The best way to
describe this direct method is to look at the following example.
www.soran.edu.iq
Example:
The combustion of sulfur occurs according to the following thermo
chemical equation:
S (rhombic) + O2(g) → SO2(g)
ΔH°rxn = - 296 kJ.
What is the enthalpy of formation of (SO2(g)).
Solution:
Step(1): recall that the enthalpy of reaction carried out under
standard-state conditions is given by
ΔH°rxn = ( (Σn ΔH°f (products) –( Σm ΔH°f (reactants) )
ΔH°rxn = [ΔH°f (SO2)(g) ] – [ΔH°f (S) + ΔH°f (O2 ) (g) ]
www.soran.edu.iq
Step(2): recall that the standard enthalpy of formation of any
element in its most stable form is zero. Therefore, ΔH°f of (S) =0
and ΔH°f of (O2 ) (g) = 0.
ΔH°rxn = [ΔH°f (SO2)(g) ] – [ΔH°f (S) + ΔH°f (O2 ) (g) ]
-296 kJ = [ΔH°f (SO2)(g) ] –[ 0+0]
ΔH°f (SO2)(g) = -296 kJ/ mol (SO2).
Note: you should recognize that this chemical equation as
written meets the definition of a formation reaction. Thus,
ΔH°rxn is ΔH°f of (SO2) (g).
www.soran.edu.iq
Indirect method of calculating the standard enthalpy of
formation, (Hess's law):
Hess's law, states that when reactants are converted to
products, the change in enthalpy is the same wither the reaction
takes place in one step on in a series of steps.
This means that if we can break down the reaction of interest
into a series of reactions for which (ΔH°rxn) can be measured , we
can calculate (ΔH°rxn) for the overall reaction. Let's look at an
example:
Example:
For the following heats of combustion with fluorine, calculate
the enthalpy of formation of methane , (CH4):
www.soran.edu.iq
(a) CH4 (g) + 4F2 (g) → CF4 (g) + 4HF (g)
(b) C(graphite) + 2F2(g) → CF4(g)
( c ) H2 (g) + F2 (g) → 2 HF (g)
ΔH°rxn= - 1942 kJ.
ΔH°rxn= - 933 kJ.
ΔH°rxn= - 542 kJ.
Solution:
Step (1): the enthalpy of formation of methane can be
determined from the following equation.
C(graphite) + 2H2 (g) → CH4(g)
ΔH°rxn= ?
If we can calculate (ΔH°rxn) for this reaction, we can
calculate ΔH°f (CH4) because the enthalpies of formation of (
C ) and ( H2(g) ) are zero. Why ? Remember that the standard
enthalpy of formation of any element in its most stable form
is zero.
www.soran.edu.iq
Step (2): since we want to obtain one equation containing only
(C,H2 , and CH4), we need to eliminate ( F2, CF4 , and HF), from
the first three equation (a-c). first, we note that equation (a)
contains methane (CH4), on the reactant side. Let's reverse (a)
to get (CH4) on the product side.
CF4 (g) + 4HF (g) →CH4 (g) + 4F2 (g) ΔH°rxn= + 1942 kJ.
Note : ΔH°rxn changed sign when reversing the direction of the
reaction.
www.soran.edu.iq
CF4 (g) + 4HF (g) → CH4 (g) + 4F2 (g) ΔH°rxn= + 1942 kJ.
C(graphite) + 2F2(g) → CF4(g)
ΔH°rxn= - 933 kJ.
2H2 (g) + 2F2 (g) → 4HF (g)
ΔH°rxn= - 1084 kJ.
C(graphite) + 2H2 (g) → CH4(g)
ΔH°rxn= - 75 kJ.
Step (3): since the above equation represents the synthesis of (CH4)
from its elements, we have.
www.soran.edu.iq
ΔH°rxn = [ΔH°f (CH4)] – [ΔH°f (C) + 2ΔH°f (H2)]
-75 kJ = [ΔH°f (CH4)] – [0 + 0]
ΔH°f (CH4) = -75 kJ / mol (CH4).
The first law of thermodynamics:
Applying the first law of thermodynamics:
The first law of thermodynamics states that energy can be
converted form one form to another, but cannot be created or
destroyed.
ΔEsys + ΔEsurr = 0
www.soran.edu.iq
Where:
The subscripts '' sys'' and '' surr'' denote system and surroundings,
respectively.
ΔE = q + w
Where:
ΔE is the change in internal energy of the system.
q is the heat exchange between the system and
surroundings.
w is the work done on (or by ) the system.
www.soran.edu.iq
Using the sign convention for thermo chemical processes, (q
is positive) for an endothermic process and (q is negative) for
an exothermic process. For work, (w is positive ) for (work
done on the system by the surroundings) and (w is negative
for work done by the system on the surroundings).
www.soran.edu.iq
Example:
A system does (975 kJ) of work on its surroundings while at the
same time it absorb ( 625 kJ) of heat. What is the change in energy,
ΔE, for the system.
Solution:
To solve this problem, you must make sure to get the sign
convention correct. The system does work on the surroundings; this
is an energy-depleting process.
w = - 975kJ.
The system absorbs (625 kJ) of heat. Therefore, the internal energy
of the system would increase.
q = + 625kJ.
ΔE = q + w = 625kJ + ( - 975kJ) = - 350 kJ.
www.soran.edu.iq