Polyatomic Ions
advertisement

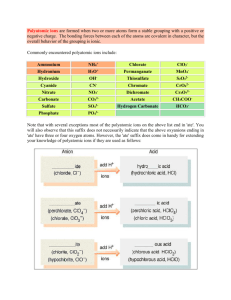
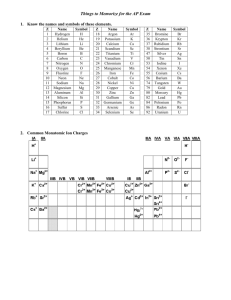
Unit 1: Chemistry Basics 1.1 Polyatomic Ions Taking Notes in AP Chem • I will give you a print out of the slides. • This is exactly how it will go in college. • Anything I write, you should write. • For the AP exam…. While they give us a periodic table they do not give us a list of polyatomic ions. • We must memorize the name, formula and charge for the common ones Polyatomic Name NH4+ NO2NO3SO32SO42HSO4OHCNOCNSCNPO43HPO42H2PO4CO32HCO3ClOClO2ClO3ClO4BrOBrO2BrO3BrO4IOIO2IO3IO4C2H3O2MnO4Cr2O72CrO42O22C2O42NH2BO33S2O32- Ammonium Nitrite Nitrate Sulfite Sulfate Hydrogen sulfate (bisulfate) Hydroxide Cyanide Cyanate Thiocyanate Phosphate Hydrogen phosphate Dihydrogen phosphate Carbonate Hydrogen carbonate (bicarbonate) Hypochlorite Chlorite Chlorate Perchlorate Hypobromite Bromite Bromate Perbromate Hypoiodite Iodite Iodate Periodate Acetate Permanganate Dichromate Chromate Peroxide Oxalate Amide Borate Thiosulfate Here are some resources to help you • • • • Tricks for memorizing polyatomics http://www.youtube.com/watch?v=jcKR9U4Ixlk http://www.youtube.com/watch?v=Iva9ISbxbOw http://www.purposegames.com/game/commonpolyatomic-ions-quiz • http://www.proprofs.com/quizschool/story.php?title=polyatomic-ions_2 • http://www.youtube.com/watch?v=vOoyLbG7afs Nick the Camel ate a Clam for Supper in Phoenix • Count consonants for # of oxygen atoms • Count vowels for negative charge of polyatomic • This trick only works for the following 5 polyatomic ions that end in ate Nick the Camel ate a Clam for Supper in Phoenix • Nick – 3 consonants = – 1 vowel = Hi I’m Nick Nick the Camel ate a Clam for Supper in Phoenix • Camel – 3 consonants = – 2 vowel = Nick the Camel ate a Clam for Supper in Phoenix • Clam – 3 consonants = – 1 vowel = Nick the Camel ate a Clam for Supper in Phoenix • Supper – 4 consonants = – 2 vowels = Nick the Camel ate a Clam for Supper in Phoenix • Phoenix – 4 consonants = – 3 vowels = Other helpful hints… • There are only 2 polyatomic ions that we need to memorize that have 3- charge – Phosphate PO43– Borate BO33We can use “nick the camel” to get phosphate If we look at PT you know boron • Halogens group (column) #7A for 1- ions Polyatomic Ions that start with Cl Br I All make 1- polyatomic ions …. Combining rules for halogens Polyatomic Ions that start with Cl, Br, I All make 1- polyatomic ions And if “ate” is the base All of these have 3 oxygens in “ate” form ClO3- = chlorate BrO3- = bromate IO3- = iodate Think of “ate” for as the “normal poly form ” • So if it changes to “ite” – that means one less oxygen but no charge change • SO42- sulfate SO32- sulfite • “Hypo” prefix means one less …so one less oxygen and no charge change • “Per” prefix mean one more…so one more oxygen and no charge change ClO- ClO2- ClO3chlorate ClO4- Hydrogen in the name • Means literally they added one hydrogen atom • Since hydrogen ions are often 1+ charge • So.. The overall charge must change by adding 1+ CO32HCO3I** old naming system instead of hydrogen they added prefix ”bi” to indicate one hydrogen atom was added bicarbonate HCO3- Dihydrogen • Means literally they added two hydrogen atom • Since hydrogen ions are often 1+ charge • So.. The overall charge must change by adding 1+ + 1+ = 2+ PO43HPO42- H2PO4** note this only can happen if “base” is 3- charge This is the only dihydrogen one you need to know Thio… • Thio = means take away one Oxygen and replace it with one Sulfur and don’t change the charge • Sulfate = • Thiosulfate = • Cyanate = • Thiocyanate = That leaves 10 more out of the 36 on the list • Ammonium • Dichromate • Hydroxide • Chromate • Cyanide • Peroxide • Acetate • Permanganate • Oxalate • Amide