ChemistryName: Ms. Boon Period: ______ Date: Thermochemistry
advertisement

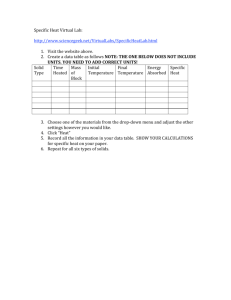
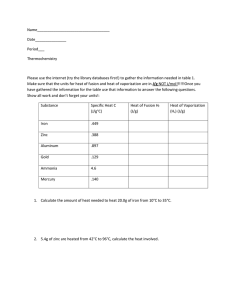
Chemistry Ms. Boon Name: _________________________________ Period: ______ Date: _______________ Thermochemistry Notes 2 Catalyst: Analyze heat flow in the systems shown on the board here. Video Demonstration - Focus Question: Are these reactions exothermic or endothermic? How do you know? States of Matter Vocabulary Review: Use your notes to complete the chart AND bubbles. PHASE CHANGE Solid to Liquid Liquid to Solid Liquid to Gas Gas to Liquid Solid to Gas Gas to Solid CHEMISTRY TERM ENDOTHERMIC OR EXOTHERMIC? The temperature at which a substance turns from a solid to a liquid is called the … The temperature at which a substance turns from a liquid to a gas is called the … Notes: Specific Heat (aka heat capacity or specific heat capacity) Specific heat is the amount of __________________ that must be transferred as ______________ to raise the temperature of 1 gram (g) of a substance by 1 Kelvin (K). The term used for specific heat is a lowercase “c”. The units for specific heat are Joules/gram*Kelvin or __________. Different substances have ______________________ specific heats. Table 1: Specific Heats at Room Temperature Substance Specific Heat (J/g*K) Air 1.01 2. What substance has the lowest specific heat? Aluminum 0.897 Cadmium 0.232 3. Which substance requires more heat energy to increase its Calcium 0.647 temperature: calcium or gold? Carbon (graphite) 0.709 Chromium 0.449 4. A substance with a high specific heat requires (more/less) heat Copper 0.385 energy to raise the temperature of a 1 g sample by 1 K than a Gold 0.129 substance with a low specific heat. Granite 0.79 Specific Heat Calculations Iron 0.449 If we know the specific heat of a substance, we can calculate the amount of Lead 0.129 heat energy required to raise the temperature of a sample of a substance by a Neon 1.030 certain amount. Nickel 0.444 Platinum 0.133 The formula: Silicon 0.705 Silver 0.235 Water 4.18 Zinc 0.388 Specific Heat Data. Use the table to answer the following questions. 1. What substance in the table as the highest specific heat? Chemistry Ms. Boon Name: _________________________________ Period: ______ Date: _______________ Example 1: A 100.0 g sample of water was heated and the temperature increased by 10 K. How much heat did the water absorb? Heat Transfer Calculations Example 2: A 10.0 g sample of aluminum was heated from 300 K to 325 K. How much heat was absorbed by the aluminum? (1) A 2.0 g sample of lead was heated and the temperature increased by 100 K. How much heat did the lead absorb? (2) A 50.0 g sample of carbon was heated from 276 K to 376 K. How much heat was absorbed by the carbon? (3) A 40.0 mL sample of water (with a mass of 40.0 g) was poured into the LA river and its temperature dropped from 35 °C to 5 °C. How much heat was released? (4) A 20.0 g sample of silicon was heated and the temperature increased by 110 K. How much heat did the silicon absorb? (5) A 500.0 mg sample of silver was heated from 276 K to 376 K. How much heat was absorbed by the silver? (6) A 40.0 g gold nugget was dropped in the Merced river and its temperature dropped from 35 °C to 5 °C. How much heat was released? (7) A 23.0 g sample of water was heated and the temperature increased by 50 K. How much heat did the water absorb? (8) A 60.0 g sample of chromium was heated from 300 K to 350 K. How much heat was absorbed by the chromium? (9) 100 g of water was cooled from 370 K to 273 K. How much heat was released? What will happen to the water if it continues to cool? Example 3: A 0.5 g platinum ring was dropped in the snow and its temperature dropped from 35 °C to 0 °C. How much heat was released?