Topic 3 - MrSimonPorter
advertisement

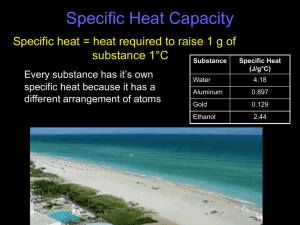
Today’s lesson • Demonstrate understanding of the term thermal capacity • Describe an experiment to measure the specific heat capacity of a substance • Recall and use the equation: Energy = mass x specific heat capacity x change in temperature Imagine if…….. Two beakers containing different amounts of water were heated by identical heaters for an equal amount of time. Imagine if…….. The beaker with less water gets hotter. WHY? Imagine if…….. Two beakers, one containing water and one containing kerosene (equal masses) were heated by identical heaters for an equal amount of time. The beaker containing the kerosene would be hotter! WHY? Since the amount of heat energy supplied is the same to both substances, it seems that different substances require different amounts of heat energy to cause the same temperature rise. Why are thermal capacities different? • When a substance is heated, its internal energy increases (potential and kinetic). The stronger the force between the particles in the substance, the more heat energy goes into potential energy (and less into kinetic), so the temperature rise is less than in substances with little force between particles. Obviously the more particles there are too, the more heat energy can be absorbed. Specific heat capacity Specific heat capacity is the amount of energy needed to raise the temperature of one kilogram of a substance by 1°C Specific heat capacity of water = 4186 J/kg/°C Specific heat capacity of kerosene = 2010 J/kg/°C Specific heat capacity of mercury = 140 J/kg/°C Calculations using S.H.C. Energy absorbed = Mass x Specific Heat capacity x Temp rise J kg J/kg/°C E = mcΔT °C For example 0.5 kg of olive oil is heated until its temperature rises by 120 K. If the specific heat capacity of olive oil is 1970 J/kg/°C, how much heat energy was used? Energy absorbed = Mass x Specific Heat capacity x Temp rise Energy absorbed = 0.5 x 1970 x 120 Energy absorbed = 118200 J An analogy: Water and wetness “This analogy is one of my ideas!” Richard Feynmann – Nobel prize winning Physicist, lock-picker and bongo player Two towels – same size/mass • You can add the same amount of water (heat), but the cheaper towel will be “wetter” (temperature). They have different capacities for absorbing water Investigation time! Let’s do an experiment to measure specific heat capacities thermometer immersion heater solid block Measuring SHCs • Energy put IN to metal = voltage x current x time • Energy = mass x specific heat capacity x temp rise voltage x current x time = mass x specific heat capacity x temp rise Specific heat capacity = (voltage x current x time)/(mass x temp rise) Let’s try some questions!