Classification of Matter
advertisement

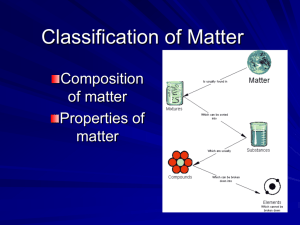
Classification of Matter Chp 15 Section 1 Composition of Matter Classification of Matter Composition of Matter slides 3-34 Properties of Matter slides 35-60 Composition of Matter Chp 15 Section 1 Composition of Matter What are substances & mixtures How to identify elements & compounds The difference between solutions, colloids, & suspensions Composition of Matter Properties of materials can be used to classify them into categories. Composition of Matter Properties of materials can be used to classify them into categories. Materials are either pure substances or a mixture of substances. Composition of Matter Properties of materials can be used to classify them into categories. Materials are either pure substances or a mixture of substances. A substance is a type of matter that is always made of the same thing(s). Composition of Matter Properties of materials can be used to classify them into categories. Materials are either pure substances or a mixture of substances. A substance is a type of matter that is always made of the same thing(s). Substances are either an element or a compound. Composition of Matter A substance is an element if all the atoms in it are the same. About 90 elements are found on Earth; another 20+ have been made in laboratories but are unstable. Composition of Matter A Compound is a substance with two or more elements that are combined in a fixed proportion. Composition of Matter A Compound is a substance with two or more elements that are combined in a fixed proportion. Water is made of elements hydrogen & oxygen in a 2:1 proportion. Composition of Matter A Compound is a substance with two or more elements that are combined in a fixed proportion. Water is made of elements hydrogen & oxygen in a 2:1 proportion. A molecule is the smallest particle of a compound that has all the properties of the compound. Composition of Matter Chalk contains calcium, carbon, and oxygen in a 1:1:3 ratio in each molecule. Composition of Matter Chalk contains calcium, carbon, and oxygen in a 1:1:3 ratio in each molecule. Elements combined in compounds often look very different; for example, silvery metallic sodium and the greenish-yellow poisonous gas chlorine combine to make sodium chloride or table salt (NaCl). Composition of Matter A mixture is a material made up of two or more substances that can be easily separated. Composition of Matter A mixture is a material made up of two or more substances that can be easily separated. In a heterogeneous mixture different materials can be identified easily. Composition of Matter A mixture is a material made up of two or more substances that can be easily separated. In a heterogeneous mixture different materials can be identified easily. Granite, pizza and concrete are some examples of heterogeneous mixtures. Also, permanent press fabric of polyester and cotton (microscope)… Composition of Matter A homogeneous mixture contains two or more substances blended evenly throughout so that you can’t see different substances in it. Composition of Matter A homogeneous mixture contains two or more substances blended evenly throughout so that you can’t see different substances in it. Examples include soft drinks in an unopened bottle; however, it becomes heterogeneous when you pour it and the carbon dioxide escapes as bubbles Composition of Matter Vinegar is another homogeneous mixture which contains acetic acid mixed with water. Composition of Matter Vinegar is another homogeneous mixture which contains acetic acid mixed with water. Homogeneous mixtures such as soft drinks and vinegar are also called solutions. Composition of Matter Vinegar is another homogeneous mixture which contains acetic acid mixed with water. Homogeneous mixtures such as soft drinks & vinegar are also called solutions. A solution is a homogeneous mixture of particles so small that they cannot be seen with a microscope & will not settle to the bottom of their container. Composition of Matter Solutions stay constantly and evenly mixed. has mass & takes up space Composition definite Two or more kinds of atoms One kind of atom composition variable Unevenly mixed Evenly mixed; a solution Matter has mass & takes up space Substance Composition definite Compound Two or more kinds of atoms Element One kind of atom Mixture composition variable Heterogeneous Unevenly mixed Homogeneous Evenly mixed; a solution Composition of Matter A colloid is a special type mixture with particles that are larger than those in solutions, but not heavy enough to settle to the bottom of their container. Composition of Matter A colloid is a special type mixture with particles that are larger than those in solutions, but not heavy enough to settle to the bottom of their container. Milk contains water, fat & proteins in different proportions with large particles. Composition of Matter A colloid is a special type mixture with particles that are larger than those in solutions, but not heavy enough to settle to the bottom of their container. Milk contains water, fat & proteins in different proportions with large particles. Paint is a liquid colloid; fog is a gas colloid; smoke is solids suspended in air. Do colloids and solutions look the same? Fog looks white because its particles are large enough to scatter light. Some shampoos & gelatins are colloids called gels that look almost clear. Do colloids and solutions look the same? Fog looks white because its particles are large enough to scatter light. Some shampoos & gelatins are colloids called gels that look almost clear. You identify colloids by shining a beam of light through it: you cannot see it in a solution but you can see it in a colloid because the large particles scatter light (Tyndall effect). What are suspensions? Some mixtures are neither solutions nor colloids, for example, muddy pond water. A suspension is a heterogeneous mixture containing a liquid in which you can see particles settle. Rivers are natural examples of suspension; moving quickly through narrow channels they pick up soil which then settles out when the water slows. Comparing Solutions, Colloids, & Suspensions Description Solutions Colloids Suspensions Settle upon No standing? No Yes Separate using filter paper? Particle size No No yes 0.1-1nm 1-100 nm >100 nm Scatter light? No Yes yes Matter Give an example of each type of matter Substance Compound Element Mixture Heterogeneous Homogeneous Matter anything Give an example of each type of matter. Substance wood Mixture pizza Compound water Heterogeneous granite Element Homogeneous Carbon vinegar Properties of Matter Chp 15 Section 2 Properties of Matter To identify substances using physical properties Differences between physical and chemical changes How to identify chemical changes The law of conservation of mass Properties of Matter A physical property is a feature or characteristic that describes an object or substance such as color, shape, size, density, melting point & boiling point. Properties of Matter A physical property is a feature or characteristic that describes an object or substance such as color, shape, size, density, melting point & boiling point. Some physical properties describe behavior of material such as magnetic, easily bent, malleable, flows easily, viscous (thick liquid). Properties of Matter Physical properties can be used to separate materials: Sifting in gem mining Magnetism to separate metal Seeds from fruit Physical Change When a substance freezes, boils, evaporates, or condenses, it undergoes a physical change. Physical Change When a substance freezes, boils, evaporates, or condenses, it undergoes a physical change. A physical change is a change in size, shape or state of matter. Heat might be added or removed but other properties never change like density, specific heat, boiling point and melting point. What is distillation? Distillation is the process of separating substances in a mixture by evaporating a liquid & condensing its vapor. What is distillation? Distillation is the process of separating substances in a mixture by evaporating a liquid & condensing its vapor. The liquid is heated and its vapor is cooled until it condenses. A solid material is left behind. Salt water can be made into drinking water this way. Distillation (continued) Liquids with different boiling points can be distilled. Vapors of the liquid with the lowest boiling point form first & are collected. As the temperature increases the second liquid boils, condenses & is collected. Natural oils such as mint are distilled. Chemical Properties & Changes A chemical property is a characteristic of a substance that indicates whether it can undergo a change that results in a new substance. When a substance burns, there is a chemical change. Flammability is a chemical property Chemical Properties & Changes A chemical change results in a new substance indicated by be smell, rust, heat, light, or sound. Burning and rusting are chemical changes because new substances are formed. Chemical Properties & Changes You can separate substances using a chemical change. Example: cleaning tarnish (silver sulfide) off silver with another chemical reaction using warm water, baking soda, and aluminum foil. Chemical Properties & Changes Weathering shapes Earth’s surface Rocks split, rivers carve deep canyons, sand dunes shift, and interesting formations develop in caves. Are these changes physical or chemical? Weathering Physical Weathering Weathering Physical Weathering Weathering Chemical Weathering Chemical weathering The most common types of chemical weathering are oxidation, hydrolysis and carbonation. Chemical weathering The most common types of chemical weathering are oxidation, hydrolysis and carbonation. Limestone which is mostly calcium carbonate dissolves in slightly acidic water to create caves and rock formations. Law of Conservation of Mass The mass of all substances that are present before a chemical change equals the mass of all substances that remain after the change. Law of Conservation of Mass The mass of all substances that are present before a chemical change equals the mass of all substances that remain after the change. When a log burns, you see smoke and light, feel heat, and note a change in appearance indicating a chemical change is taking place. Calculate If a 2-kg log is burned, what is the mass of the ash, smoke, and carbon dioxide produced by the chemical change? Calculate If a 2-kg log is burned, what is the mass of the ash, smoke, and carbon dioxide produced by the chemical change? According to the Law of Conservation of Mass, the mass of everything left would be 2-kg.