Chapter 9 Molecular Geometry and Bonding Theories
advertisement

Chemistry 100
Chapter 9
Molecular Geometry and Bonding
Theories
Molecular Geometry
The three-dimensional arrangement
of atoms in a molecule molecular
geometry
Lewis structures can’t be used to
predict geometry
Repulsion between electron pairs
(both bonding and non-bonding)
helps account for the molecular
structure!
The VSEPR Model
Electrons are negatively charged, they want to
occupy positions such that electron
Electron interactions are minimized as much as possible
Valence Shell Electron-Pair Repulsion Model
treat double and triple bonds as single domains
resonance structure - apply VSEPR to any of them
formal charges are usually omitted
Four Electron Domains – Three
Different Geometries
Replacement of bonding domains (B)
with nonbonding domains (E)results
in a different molecular geometry.
AB4
AB3E
AB2E2
Molecules With More Than One
Central Atom
We simply apply VSEPR to each ‘central atom’
in the molecule.
• Carbon #1 –
tetrahedral
• Carbon #2 – trigonal
planar
Dipole Moments
The HF molecule has a bond dipole – a charge
separation due to the electronegativity
difference between F and H.
The shape of a molecule and the magnitude of
the bond dipole(s) can give the molecule an
overall degree of polarity dipole moment.
+H-F
Homonuclear diatomics no dipole moment
(O2, F2, Cl2, etc)
Triatomic molecules (and greater). Must look
at the net effect of all the bond dipoles.
In molecules like CCl4 (tetrahedral) BF3
(trigonal planar) all the individual bond dipoles
cancel no resultant dipole moment.
Bond Dipoles in Molecules
More Bond Dipoles
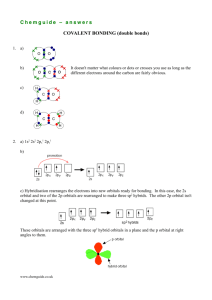
Valence Bond Theory and
Hybridisation
Valence bond theory
description of the covalent bonding and
structure in molecules.
Electrons in a molecule occupy the
atomic orbitals of individual atoms.
The covalent bond results from the
overlap of the atomic orbitals on the
individual atoms
The Bonding in Diatomic
Molecules
Hydrogen molecule
Hydrogen Chloride
a single bond between the
two H 1s orbitals
a bond
a single bond from the
overlap of the Cl 3p orbital
with the H 1s orbital
Chlorine molecule
a single bond from the
overlap of the Cl 3p
orbitals
Hybrid Atomic Orbitals
Look at the bonding picture in
methane (CH4).
• Bonding and geometry in polyatomic molecules
may be explained in terms of the formation of
hybrid atomic orbitals
• Bonds overlap of the hybrid atomic orbitals on
central atoms with appropriate half-filled atomic
orbital on the terminal atoms.
The CH4 Molecule
The Formation of the sp3 Hybrids
Mix 3 “pure” p
orbitals and a
“pure” s orbital
form an sp3
“hybrid” orbital.
Rationalize the
bonding around the
C central atom.
sp2 Hybridisation
Examine BH3
(a trigonal planar molecule)
sp Hybridisation
Examine BeF2 (a linear molecule).
These sp hybrid orbitals have an
angle of 180 between them.
A Linear Molecule
The BeF2 molecule
Double Bonds
Look at ethene C2H4.
Each central atom is an AB3 system,
the bonding picture must be
consistent with VSEPR theory.
Sigma () Bonds
Sigma bonds are characterized by
Head-to-head overlap.
Cylindrical symmetry of electron density
about the internuclear axis.
Pi () Bonds
Pi bonds are
characterized by
Side-to-side
overlap.
Electron density
above and below
the internuclear
axis.
Bond overlaps in C2H4
There are three
different types of bonds
[sp2 (C ) – 1s (H) ] x 4
type
[sp2 (C 1 ) – sp2 (C 2 ) ]
type
[2pz (C 1 ) – 2pz (C 2 ) ]
type
The C2H4 Molecule
The Bond Angles in C2H4
Bond angles HCH =
HCC 120.
bond is perpendicular
to the plane containing
the molecule.
Double bonds –
Rationalize by assuming
sp2 hybridization exists
on the central atoms!
Any double bond one
bond and a bond
The Triple Bond in C2H2
Bond angles HCH = HCC =
180.
bonds are perpendicular to
the molecular plane.
Triple bond one bond and
two bonds
Triple bond rationalized by
assuming sp hybridization exists on
the central atoms!
Bond Overlaps in C2H2
There are again three different types of bonds
[sp (C ) – 1s (H) ] x 2 type
[sp (C 1 ) – sp (C 2 ) ]
type
[2py (C 1 ) – 2py (C 2 ) ] type
[2pz (C 1 ) – 2pz (C 2 ) ] type
Bonding in H2O
Bonding Overlaps
[sp3(O)–1s(H)] x 2
Bond Overlaps in H2CO
There are again three different types of bonds
[sp (C) – 1s (H) ] x 2 type
[sp2 (C) – sp2 (O) ]
type
[2p (C) – 2p (O) ]
type
Key Connection – VSEPR and Valence
Bond Theory!!
sp3d Hybridisation
How can we use the hybridisation concept to
explain the bonding picture PCl5.
There are five bonds between P and Cl (all
type bonds).
5 sp3d orbitals these orbitals overlap with the
3p orbitals in Cl to form the 5 bonds with the
required VSEPR geometry trigonal bipyramid.
Bond overlaps
[sp3d (P ) – 3pz (Cl) ] x 5
type
sp3d2 Hybridisation
Look at the SF6 molecule.
6 sp3d2 orbitals these orbitals
overlap with the 2pz orbitals in F to
form the 6 bonds with the
required VSEPR geometry
octahedral.
Bond overlaps
[sp3d2 (S ) – 2pz (F) ] x 6
type
Notes for Understanding
Hybridization
Applied to atoms in molecules only
Number hybrid orbitals = number of atomic
orbitals used to make them
Hybrid orbitals have different energies and
shapes from the atomic orbitals from which
they were made.
Hybridization requires energy for the promotion
of the electron and the mixing of the orbitals
energy is offset by bond formation.
Delocalised Bonding
Valence bond theory –
bonding electrons have been totally associated with
the two atoms that form the bond they are
localized.
What about the bonding situation in benzene,
the nitrate ion, the carbonate ion?
Bonding in Aromatic Molecules
Benzene
C-C bonds are formed from the sp2 hybrid orbitals.
Unhybridized 2pz orbital on adjacent C atoms overlap
(bonds).
Bonding in the Benzene Molecule
The bonds extend over the whole
molecule
the electrons bonds are delocalized – they are
free to move around the benzene ring.
Resonance structures – delocalization of
the -electrons.
The Nitrate Anion
Three resonance
structures
Alternating single
and double bonds
Blend resonance
structures
Delocalized
bond over anion
backbone
Molecular Orbital (M.O.) Theory
Valence bond and the concept of the
hybridisation of atomic orbitals does not
account for a number of fundamental
observations of chemistry.
To reconcile these and other differences, we
turn to molecular orbital theory (MO theory).
MO theory – covalent bonding is described in
terms of molecular orbitals
the combination of atomic orbitals that results in
an orbital associated with the whole molecule.
Constructive and Destructive
Interference
Constructive
+
Destructive
+
ybonding = C1 ls (H 1) + C2 ls (H 2)
yanti = C1 ls (H 1) - C2 ls (H 2)
Bonding Orbital a centro-symmetric orbital
(i.e. symmetric about the line of symmetry of
the bonding atoms).
Bonding M’s have lower energy and greater
stability than the AO’s from which it was
formed.
Electron density is concentrated in the region
immediately between the bonding nuclei.
Anti-bonding orbital a node (0 electron
density) between the two nuclei.
In an anti-bonding MO, we have higher energy
and less stability than the atomic orbitals from
which it was formed.
As with valance bond theory (hybridisation)
2 AO’s 2 MO’s
Bonding and Anti-Bonding M.O.’s
from 1s atomic Orbitals
* 1s
1s
1s
Energy
1s
The MO’s in the H2 Atom
The situation for two 2s orbitals is the same!
The situation for two 3s orbital is the same.
Let’s look at the following series of molecules
H2, He2+, He2
bond order = ½ {bonding - anti-bonding e-‘s}.
Higher bond order greater bond stability.