Matter & Particle Theory - Notre Dame College School
advertisement

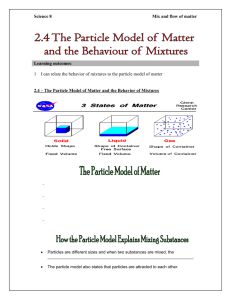
Grade 9 Science– Notre Dame College School John Dalton – the guy who came up with particle theory Agenda What is matter The Particle Theory Mixtures vs. Pure Substances Mixtures Hetrogeneous Mixtures Solution Homogeneous Mixtures Mechanical Mixture Suspension Solution Pure Substances Elements Compounds What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter IT’S ALL MATTER! The Particle Theory of Matter This describes the way to describe the structure and behaviour of matter. There are 4 principals of the particle theory written by a chemist named John Dalton. Think About it! Look at a piece of chalk If you broke a piece of chalk in half, would it still look like and behave like chalk? How small of pieces would you have to break a piece of chalk so that it isn’t chalk anymore – is that even possible? The answer is YES, because the smallest pieces of chalk are made up of particles and the particle theory explains how this works! The Particle Theory of Matter 1. All Matter is made up of very tiny objects called particles. These particles are VERY tiny – too small to be seen with any regular light microscope The Particle Theory of Matter 2. All Particles have spaces between them. These size of these spaces determine the state of the matter The Particle Theory of Matter 3. Particles present in matter are always in motion. In a solid, they vibrate together In a liquid, they stay close together but slide along each other In a gas, they bounce and move in all directions! The Particle Theory of Matter 4. The particles in a substance attract each other. The degree that particles are attracted to each other is different in difference substances, Take a guess! Which substance do you think has the strongest attractions? Which has the weakest? Iron Water Oxygen The Particle Theory of Matter Recap: Here they are all again All Matter is made up of very tiny objects called particles. 2. All Particles have spaces between them. 3. Particles present in matter are always in motion. 4. The particles in a substance attract each other. 1. Pure Substances vs. Mixtures All matter can be divided into two big categories: PURE SUBSTANCES and MIXTURES. Pure substances are made up of one type of element or compound Mixtures are a combination of pure substances (2 or more types of particles) Versus Pure Substances Pure substances are made up of only ONE type of particle Pure substances are in the form of either elements or compounds. Elements Elements are the smallest and “Purest” forms of particles They cannot be broken down further by ordinary means (such as simple reactions, heat, or electricity) They are only made up of one type of atom (one type of particle Examples are Oxygen, Calcium, Iron, Carbon and Helium Compounds A compound is made up of only one type of particle, Called a molecule Compounds are made up of many molecules that are held together by chemical bonds! Hydrogen Oxygen Hydrogen Water is a molecule because it is made out of two types of atoms (oxygen and hydrogen) Water is a compound because it has many molecules of one type (H2O = Water) Mixtures A mixture is a combination of two or more different substances (different types of particles). Mixtures can be divided into 2 big categories: Heterogeneous Mixtures Homogeneous Mixtures Heterogeneous Mixtures Made up of two or more particles where the different particles are easy to see and separate. Can be divided into two categories: Mechanical mixtures Suspensions An Oreo cookie is a heterogeneous mixture Mechanical Mixtures Particles that are in the same container but can be easily separated. Made up of several distinct parts A lasagne, parfait, and a cookie are both examples of mechanical mixtures THINK ABOUT IT! What are some of the parts of these mechanical mixtures? Suspensions Small particles of one substance float in another substance Salad Dressing, glittery nail polish, and yogurt are examples of suspensions Homogeneous Mixtures A homogenous mixture occurs when 2 or more particles blend together and cannot be seen separately Solutions are homogeneous mixtures! Solutions A mixture of 2 or more things where one substance dissolves Kool-Aid and tea are solutions because you cannot see the flour crystals floating in the kool-aid, and you cannot see the tea floating in the tea when the bag is removed Kool-Aid and tea are examples of solutions