Phase Diagram Worksheet
advertisement
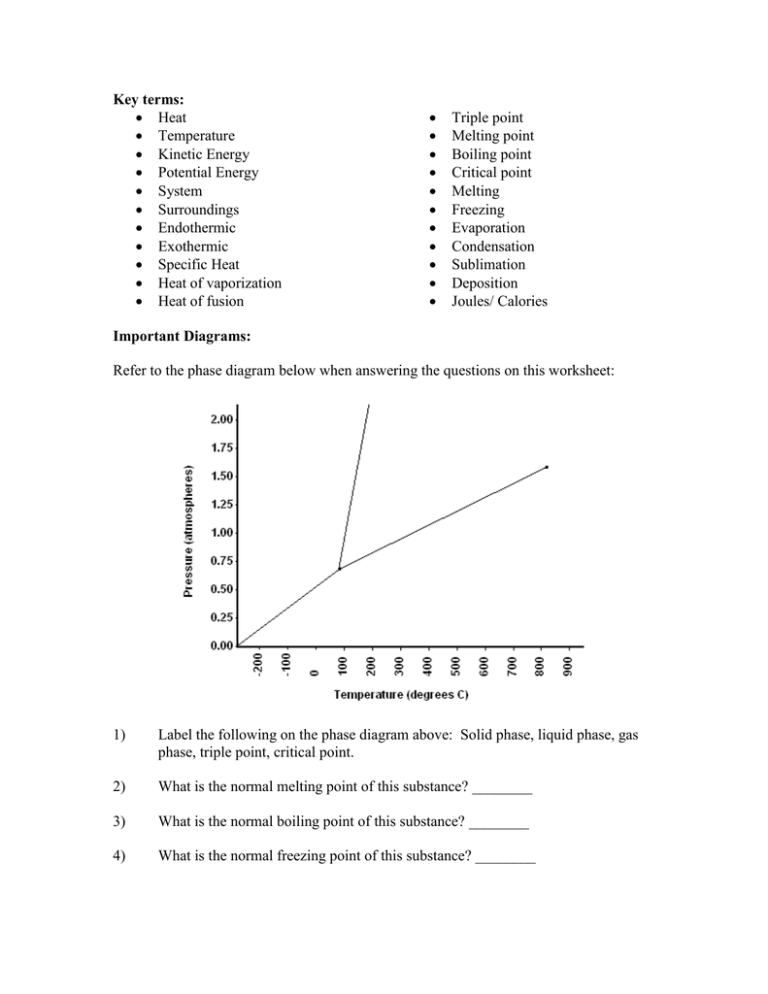
Key terms: Heat Temperature Kinetic Energy Potential Energy System Surroundings Endothermic Exothermic Specific Heat Heat of vaporization Heat of fusion Triple point Melting point Boiling point Critical point Melting Freezing Evaporation Condensation Sublimation Deposition Joules/ Calories Important Diagrams: Refer to the phase diagram below when answering the questions on this worksheet: 1) Label the following on the phase diagram above: Solid phase, liquid phase, gas phase, triple point, critical point. 2) What is the normal melting point of this substance? ________ 3) What is the normal boiling point of this substance? ________ 4) What is the normal freezing point of this substance? ________ 6) At what temperature do the gas and liquid phases become indistinguishable from each other? ________ A sample of water is heated from a liquid at 40oC to a gas at 110oC. a) On the heating curve diagram provided above, label each of the following regions: Liquid, only Gas, only Phase change b) For section QR of the graph, state what is happening to the water molecules as heat is added. c) For section RS of the graph, state what is happening to the water molecules as heat is added. Math Concepts: Specific Heat 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron. 2. To what temperature will a 50.0 g piece of glass raise if it absorbs 5275 joules of heat and its specific heat capacity is 0.50 J/g°C? The initial temperature of the glass is 20.0°C. Heating and Cooling Curves: Refer to the following values to help solve the problems. c(ice) = 2.06 J/gC, c(H2O) = 4.184 J/gC, H(fus) for H2O is 334.J/g, c(steam) = 1.87 J/gC, H(vap) for H2O is 2260 J/g 1. Calculate the amount of heat needed to convert 190.0 g of liquid water at 18 ºC to steam at 100. ºC. (Sketch out the curve first!). 2. Calculate the amount of heat needed to convert 96 g of ice at –24 ºC to water at 28 ºC. (Sketch out the curve first!!)