AP Chemistry – Snow Day 9: Periodic Trends
advertisement

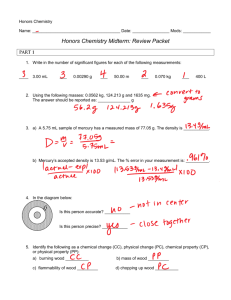
AP Chemistry – Snow Day 9: Periodic Trends - Key Name __________________________________ Period _____ 1. Explain why a calcium atom is larger than a zinc atom. Zinc and calcium atoms each have 4 energy levels however a zinc atom has a greater effective nuclear charge because it has 30 protons in its nucleus whereas a calcium atom only has 20. 2. Explain why the ionic radius of N3¯ is larger than that of O2¯. Each of these ions have 10 electrons however oxide has a greater effective nuclear charge with 8 protons in its nucleus as compared to 7 protons in the nucleus of nitride. 3. Why does potassium have a lower first-ionization energy than lithium? The first electron removed from potassium is in the 4th energy level in a 4s sublevel which is much further from the nucleus and better shielded than the first electron removed from lithium which is in the 2nd energy level in a 2s sublevel. Therefore the effective nuclear charge of potassium is lower and it takes less energy to remove the 4s electron compared to the 2s electron. 4. Why is the lattice energy of CaO(s) greater than the lattice energy for K2O(s)? The lattice energy for each of these ionic solids is based on the strength of the ionic bonding in each. The ionic bonds are stronger in CaO than in K2O because Ca is a 2+ ion and K is a 1+ ion. Therefore the product of the ionic charges is greater in CaO than in K2O. 5. Why do the melting points of the halogens increase as we move down the group? Halogen molecules are held in place by dispersion (van der Waals) forces: bonds due to temporary dipoles caused by polarization of electron clouds. As molecules increase in size (F2 to I2), the larger electrons clouds are more readily polarized, and the attractive forces between molecules (and melting points) increase. 6. Element Q has the following first three ionization energies [in kJ/mole]: I1 Q I2 I3 496 4568 6920 What is the formula for the most likely compound of element Q with chlorine? Explain the choice of formula on the basis of the ionization energies. The formula is QCl A very high second ionization energy indicates that there is only one valence electron.