Experiment 23:
advertisement

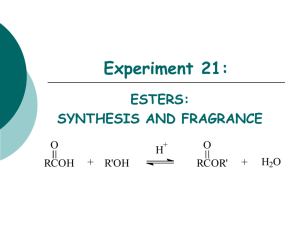
Experiment 21: ESTERS: SYNTHESIS AND FRAGRANCE O O + H2SO4 OH OH acetic acid + isoamyl alcohol O isoamyl acetate H2O Objectives: To synthesize an ester from acetic acid with isoamyl alcohol under reflux by Fischer esterification. To purify the product through acid-base extraction and simple distillation. To identify and analyze the purity of the product using GC analysis. To characterize the reactant and product using NMR and IR spectra. Before coming to lab… Review these techniques: Reflux Acid-Base Extraction Simple distillation Drying over MgSO4 GC Analysis FISCHER ESTERIFICATION Fischer esterification is an acid catalyzed nucleophilic acyl substitution. Net effect is the replacement of an –OH of a carboxylic acid with the –OR of an alcohol to produce an ester. O R O H + H O C O R' H + R O C R' + H O It is an equilibrium reaction with an unfavorable Keq, thus we can improve product yield in several ways: Use an excess of the alcohol reactant. Use an excess of the carboxylic acid reactant. Remove water as it forms. H FISCHER ESTERIFICATION H O C H3C CH3 O + OH H OSO3H C H3C + OH H OH C CH3 • Carboxylic acids are not reactive enough to undergo nucleophilic addition directly. • By using sulfuric acid as a catalyst, the carbonyl group oxygen is protonated to give the carboxylic acid a positive charge. This makes it more reactive. MECHANISM 1. Protonation of the carbonyl oxygen activates the carboxylic acid… O C H3C 1 H CH3 O + H OSO3H + C OH H3C C OH 2 3. … yielding a tetrahedral intermediate. H 3 OH CH3 H3C OH 2. …toward nucleophilic attack by the alcohol… H C C H 3C O HO 4 5 H 4. Transfer of a proton from one oxygen to another yields a second tetrahedral intermediate… CH3 6 H H3C O H C O H3C H 6. Loss of a proton and expulsion of H2O regenerates the acid catalyst and gives the ester product. O H C O H 5…and converts the OH group into a good leaving group. CH3 H O H3C C H 3C H C O CH3 H2O H2SO4 OVERVIEW Synthesize product ester under reflux. Neutralize acetic acid with 10% NaHCO3. Extract in ether, then wash with 10% NaHCO3. Dry organic layer. Perform simple distillation to remove ether from product. Obtain final product mass, calculate % yield, prepare GC sample. EXPERIMENTAL PROCEDURE (SYNTHESIS) • Combine acetic acid, isoamyl alcohol and sulfuric acid in a 25 mL round bottom flask. CaSO4 tube • Clamp flask to ring stand and add 3 boiling chips. water out • Place water cooled condenser on top of flask, with a CaSO4 drying tube in the top. water in • Heat to reflux. Reflux 30 minutes. • Cool to room temp. • Add 10% NaHCO3 slowly to flask. 25mL heating mantle to voltage regulator iron ring EXPERIMENTAL PROCEDURE (PURIFICATION) • Transfer liquid to separatory funnel. • Rinse reaction flask with ether and transfer to separatory funnel. • Wash the organic layer with 10% NaHCO3. • Transfer the organic layer to a clean flask. 125 mL • Dry over MgSO4. • Transfer liquid to a preweighed 50 mL round bottom flask. 50mL • Clamp flask to ring stand. 50mL EXPERIMENTAL PROCEDURE (PRODUCT ISOLATION) • Set up a simple distillation apparatus, using a 25 mL round bottom as the receiving flask. •Collect all distillate that boils under 40oC. water out water in • Record distillation range, Ti-Tf. Heating Mantle • Allow reaction flask to cool to room temperature. • Reweigh 50 mL flask to determine final product mass (actual yield) and calculate % yield. • Prepare GC sample. iron ring to voltage regulator 50mL PRODUCT!!! 25mL WASTE SOLVENT Table 21.1: Experimental Results Theoretical yield (g) Actual yield (g) % yield Calculated value based on limiting reagent! (50mL RB flask + product) – (Empty 50mL RB flask) ACTUAL YIELD THEORETICAL YIELD X 100 PHYSICAL STATE, COLOR Product Appearance Table 21.2: GC Analysis Compound GC Retention Times (min) Standard Sample Area Percent Adjusted Area Percent methanol SOLVENT isoamyl alcohol REACTANT isoamyl acetate PRODUCT EXPERIMENTAL PROCEDURE (IR ANALYSIS) CH3 5 CH3CHCH2CH2OH 4 3 CH3 2 1 O 5 CH3CHCH2CH2OCCH3 4 3 2 1 6 7 Table 21.3: IR Analysis Functional Group sp3 CH stretch Base Values Acetic acid Isoamyl alcohol Isoamyl acetate Frequency (cm-1) Frequency (cm-1) Frequency (cm-1) Frequency (cm-1) 2850-3000 C-O stretch 1000-1300 OH stretch 2400-3600 C=O stretch 1640-1750 EXPERIMENTAL PROCEDURE (NMR ANALYSIS) CH3 5 CH3CHCH2CH2OH 4 3 2 CH3 1 O 5 CH3CHCH2CH2OCCH3 4 3 2 1 6 O C H3C 1 2 OH 3 7 SAFETY CONCERNS CAUTION: H2SO4 is a STRONG ACID! WASTE MANAGEMENT • All aqueous washes from the extraction can be flushed down the drain with plenty of water! • Ester product and distilled ether should be placed in the bottle labeled, “ORGANIC WASTE (Esters)”. CLEANING Clean round bottom flasks and distillation glassware with wash acetone only! Separatory funnel and all other glassware should be cleaned with soap, water, and a brush if necessary, followed by a wash acetone. DO NOT return any glassware to lab drawer dirty or wet! In-lab Question (The following question should be answered in laboratory notebook.) a. Draw the product, and a complete mechanism for its formation, of the reaction shown below. O C OH + CH3OH 0.25 g used OCH3 1.0 g used H2SO4 0.01 g used ? In-lab Question (The following question should be answered in laboratory notebook.) Based on the chemical equation shown in the previous question, calculate the theoretical yield of the product. Show calculations based on all reactants.