Unit 1 Lesson 1 - Matter and Change
advertisement
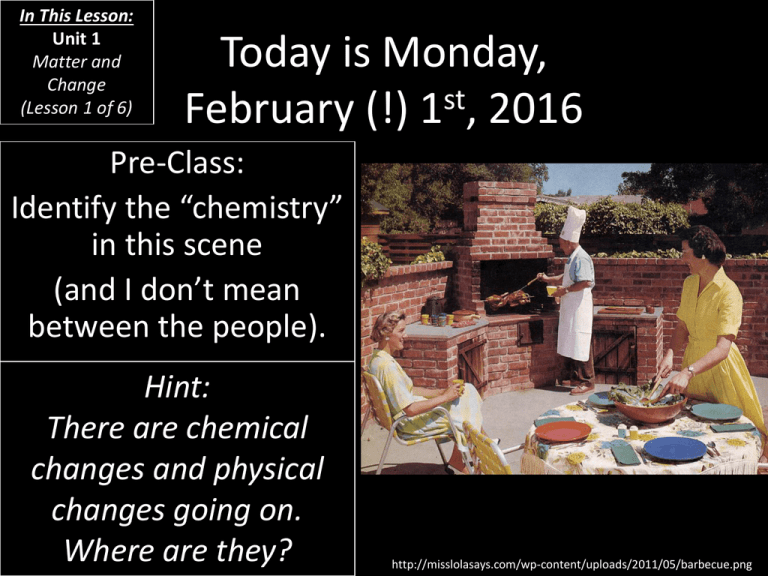
In This Lesson: Unit 1 Matter and Change (Lesson 1 of 6) Today is Monday, February (!) 1st, 2016 Pre-Class: Identify the “chemistry” in this scene (and I don’t mean between the people). Hint: There are chemical changes and physical changes going on. Where are they? http://misslolasays.com/wp-content/uploads/2011/05/barbecue.png Today’s Agenda • • • • Matter Forms of matter Changes in matter Fire? http://civilianmilitaryintelligencegroup.com/wp-content/uploads/2010/08/en-greek-fire.jpg By the end of this lesson… • You should be able to distinguish between physical and chemical changes. • You should be able to interpret a basic chemical reaction. “What’s a matter?” • First, where is this in my book? – P. 39 and following… “What’s a matter?” • What you might call “stuff,” scientists call matter. • Matter is anything that has mass and volume (takes up space). – Things that are not matter: • Love • Religion • Green – Things that are matter: • Just about everything you could touch. • Chemistry, then, is the study of matter and change. Phases of Matter • You probably learned about the phases of matter long before you even remember: – Solid – Liquid – Gas • That’s all of ‘em, right? – There’s also plasma and Bose-Einstein Condensates and a bunch of other stuff… – We’ll talk about ‘em… Phases of Matter • So that we’re all on the same page… – Solid: – Liquid: – Gas: – Plasma: Transitions • As you know, matter is not always stuck in one phase. Take water, for example. As a… – …solid, we call it ice. – …liquid, we call it water. – …gas, we call it water vapor. • And what determines which state it’s in? – Temperature (and pressure, too) • Plus, there really distinct points at which these changes occur. Matter and Change • There are two types of changes that can occur in matter, physical and chemical. – Physical changes occur when only the form of a substance has changed. • In other words, the substances are not changed into different substances. – Chemical changes occur when a substance changes into another substance (the composition changes). • Most chemical changes come along with some distinct signs (two slides). • Physical changes can be undone. • Chemical changes tend to be irreversible. Physical Changes in Matter • Going from a solid to a liquid is called: – Melting (or fusion) • Going from a liquid to a gas is called: – Evaporating (or vaporization) • Going from a gas to a liquid is called: – Condensing • Going from a liquid to a solid is called: – Freezing (or solidification) • Going from a solid to a gas is called: – Sublimation • Going from a gas to a solid is called: – Deposition Chemical Changes • You are probably witnessing a chemical change if you detect: – Color changes – Odor changes – Energy changes (as in heat) • Important: Dissolving processes sometimes give off or absorb heat. Dissolving is a physical change. – Production of gases or solids Examples of Physical and Chemical Changes • Leaves changing color – Chemical change • Cooking a burger – Chemical change • Melting an ice cube – Physical change • Rotting meat – Chemical change • Rain forming and falling – Physical change • Dissolving salt into water – Physical change More Examples of Changes • Let’s further investigate physical and chemical changes with a lab! – Chemical and Physical Changes Activity Writing Chemical Reactions • All chemical reactions have reactants and products. – The starting “ingredient(s)” are the reactants while the product(s) are the end result(s). • They are typically written like this: Reactant + Reactant Product + Product OR Reactant + Reactant Product + Product IMPORTANT NOTE: The arrow means “yields.” Writing Chemical Reactions • Examples: • H2 + O2 H2O – Reactants: Hydrogen (H2), Oxygen (O2) – Products: Water (H2O) • 2Fe + 3H2O Fe2O3 + 3H2 – Reactants: Iron (Fe), Water (H2O) – Products: Rust (Fe2O3), Hydrogen (H2) Writing Chemical Reactions • Which is the reactant? Which is the product? – 2H2O 2H2 + O2 • Reactants: – 2H2O • Products: – 2H2 – O2 More on Chemical Reactions • Scientists sometimes use symbols to describe what form the reactants or products are in: • • • • (s) means the item is a solid (l) means the item is a liquid (g) means the item is a gas (aq) means the item is aqueous (it’s dissolved but isn’t a liquid) Conservation of Mass/Matter • In any chemical reaction, mass is conserved. – In other words, the mass of the reactant(s) is the same as the mass of the product(s). – The elements on one side of the equation are the same as those on the other. – Matter cannot be created nor destroyed. • This is called the Law of Conservation of Mass. • Sometimes it is called the Law of Conservation of Matter. – Because if you’re conserving matter, you’re also conserving mass. Aside: Chemistry History • Among other things, AntoineLaurent de Lavoisier discovered something concerning the Law of Conservation of Mass. – He noticed that rusting iron gained mass. • What was the mass coming from? • So what came of Lavoisier? – He was beheaded during the French Revolution. Sacre bleu! Antoine-Laurent de Lavoisier http://upload.wikimedia.org/wikipedia/commons/6/6c/Antoine_lavoisier_color.jpg Aside: How Much Does A Soul Weigh? • Meet Dr. Duncan MacDougall. • In 1907, Dr. Mac decides to see if he can measure the mass of a soul. • Since matter can neither be created nor destroyed, Mac (or “Om,” as he was called), measured the masses of six patients as they died on the table: – Two tests were invalid. – Two showed a drop in mass, then an increase. – One showed a drop, then increase, then drop. • The first test performed registered a drop of 0.75 ounces, or 21 grams, hence the common “legend.” – MacDougall did the same tests with dogs and found no such results. http://boingboing.net/images/_images_front_picture_library_UK_dir_13_fortean_times_6877_12.jpg Conservation of Mass/Matter • Another way to look at the Law of Conservation of Mass (or Matter) is the difference between mass and weight. • Mass is the amount of matter in a substance. That doesn’t change. • Weight is the effect of gravity on an object’s mass. – You even have slightly different weights on the equator as at the pole (more at the pole, due a lower rotation rate). • Compare: – http://www.exploratorium.edu/ronh/weight/ The Law of Conservation of Mass • It’s time we had a little firsthand experience with the Law of Conservation of Mass. – Conservation of Mass Lab!









