Conservation of Mass
advertisement

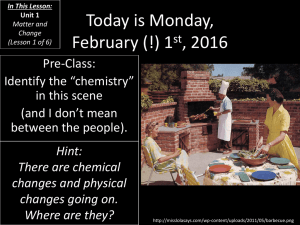
SC.10.08.04.01 Investigates the Law of Conservation of mass as it relates to physical and chemical changes (CMCS 2.3) (CAS 8.1.3) Conservation of Mass Introduction: The Law of Conservation of Mass states that in an ordinary chemical reaction, the total mass of the reactants is conserved. This means that whatever amount of reactant that you start put with, you will end up with as product. Sometimes not all of the reactant is used up, but mass is still conserved. I give you an example: I can weigh out an old-time flashcube and record the weight. I then use the flashcube to take a picture and reweigh the flashcube. The weight after the chemical reaction of the flashcube will be identical. How do I know there was a chemical reaction? The production of light and heat would prove that. Task: You have to prove conservation of mass to me in an experiment. In other words, think of something you can concoct up at home that goes through a chemical reaction and will weigh the same before and after the reaction. You must demonstrate your reaction in class on __________. You may work with one partner or alone, no bigger groups. Caution: if you partner does not come through, you are on your own. Hints: This may at first seem difficult to achieve, but remember that many reactions are used in cooking or cleaning. You also have to consider that if your reaction creates a gas, that you cannot let it escape, or your experiment will not work. Finally, I will not allow reactions that are in anyway dangerous, so sorry, but no landmines, neutron bombs, nerve gases, etc. Rubric: Advanced (30 pts): 1.Understands the reactants involved in reaction and the products formed. 2.Understands what type of reaction is involved. 3.Demonstrates that matter was conserved by weighing the reaction components before and after the reaction without mass loss. 4.Uses an original reaction (nobody else has done the same thing). 5. Can prove that a chemical reaction happened. 6. Has visual aid supporting demonstration. Proficient (20pts): 1.Understands reactants involved in reaction. 2.Understands what type of reaction is involved. 3.Demonstrates that matter was conserved by weighing the reaction components before and after the reaction, slight mass loss acceptable with explanation. 4.Uses a fairly original reaction (variation of something already seen). 5. Can prove that a chemical reaction happened. Incomplete (0 pts): Does not meet the above standards. Partnership sign-up sheets: Person 1 Person 2