I 2
advertisement

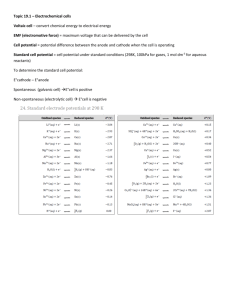
Electrochemistry: Oxidation-Reduction Reactions gaining to 2e- Zn(s) + Cu+2 (aq) Zn2+(aq) + Cu(s) loss of 2e- Copper is reduced because it goes down in charge and is the oxidizing agent Zinc is oxidized - it goes up in charge and is the reducing agent Electrochemistry Messing with electrons What is the charge on each atom +1 +1 +7 +7 -8 +3 -2 +3 KMnO4 -3 -2 +1 Cr(OH)3 +3 -2 +3 -1 =1 Fe(OH)2+1 Electrochemistry: Oxidation-Reduction Reactions By writing the oxidation number of each element under the reaction equation, we can easily see the oxidation state changes that occur 0 +1 +2 0 Zn(s) + 2H+(aq) Zn2+(aq) + H2(aq) In any oxidation-reduction reaction (redox), both oxidation and reduction must occur. Electrochemistry: Balancing Oxidation-Reduction Reactions Oxidation Number Method -3e’s X 4 = -12 4 Al(l) 0 + 3 MnO2 2Al2O3 + 3 Mn +4 -2 +4e’s X 3 = +12 +3 -2 0 Electrochemistry: Balancing Oxidation-Reduction Reactions Sample problem: +2 -2e’s X 5 = -10 +4 I2O5(s) +5 CO(g) I2(s) + 5 CO2(g) +5 +5e’s X 2 = 10 0 Half-Reaction Method : MnO4-(aq) + C2O42-(aq) Mn2+(aq) + CO2(g) (acid) Half-Reaction Method (in acid) : MnO4-(aq) + C2O42-(aq) Mn2+(aq) + CO2(g) Step 1: Divide into two half-reactions, MnO4- Mn2+ C2O42- CO2 Step 2: Balance main element MnO4- Mn2+ C2O42- 2CO2 Step 3: Balance the O: atoms by adding H2O MnO4- Mn2+ + 4H2O C2O42- 2CO2 Step 4: Balance the H atoms by adding H+ 8H+ - Mn2+ + 4H O MnO + 4 2 C2O42- 2CO2 Step 5: Balance charge with electrons +7 +2 5e- + 8H++ MnO4- Mn2+ + 4H2O -2 0 C2O42- 2CO2 + 2e- Step 6: Make the electrons balance : 10 16 2 2 8 5e- + 8H++ MnO4- Mn2+ + 4H2O 5 10 10 C2O42- 2CO2 + 2e- Step 7: Cancel and add 10e- + 16H++ 2MnO4- 2Mn2+ + 8H2O + 5C2O42- 10CO2 +10e- 16H+ + 2MnO4- + 5C2O42- 2Mn2+ + 10CO2 + 8H2O Balancing in base CN- + MnO4- CNO- + MnO2 -1 +1 3 6 + 6 3 - 3 H2O + CN CNO + 2H + 2e 6 3e-+ 2 8 4H+ + +3 2 MnO4 MnO2 4 + 2H2O 0 2H+ + 3CN- + 2MnO4- 3CNO- + 2MnO2 + H2O + 2OH2OH2H2O 1 Balancing in base Cr(OH)3 + ClO CrO4-2 + Cl2 Have at it 4Cr(OH)3 + 6ClO + 8OH- 4CrO4-2 + 3Cl2 + 10H2O Note that the activity series is simply the oxidation halfreactions of the metals ordered from the highest to lowest Using the hydrogen electrode as a reference 2H+ (1 M) + 2e- H2 (1 atm, 25 °C) E°red = 0 V Cell EMF or Voltage From the table: Flip Zn+2 + 2e- Zn red= -.76V Cu+2 + 2e- Cu red= .34V Zn Zn+2 + 2e- ox = .76V Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) E°cell = 1.10 V E ° ox + E ° red = E°cell oxidation + reduction Voltaic or Galvanic Cells Sample Problem. Draw a voltaic cell using the following equation and label all parts Cr2O72-(aq) + I-(aq) Cr3+(aq) + I2(s) Spontaneity and Extent of Redox Reactions A positive emf indicates a spontaneous process, and a negative emf indicates a nonspontaneous one. Sample Problem: Using the standard electrode potentials, determine whether the following reaction are spontaneous A. Cu(s) + 2H+(aq) Cu2+(aq) + H2(g) Cu(s) Cu2+(aq) + 2eEox = -.34V 2H+(aq) + 2e- H2(g) Ered = 0.0V Ecell = -.34V B. Cl2(g) + 2I-(aq) 2Cl-(aq) + I2(s) Cl2(g) + 2e- 2Cl-(aq) Ered = 1.36V 2I-(aq) 2e- + I2(s) Eox = -.54V Ecell = .82V EMF and Free Energy Change Any redox reaction involves free energy change (G) which also may be used as a measure of spontaneity or work (max or min.) G° = -nE ° n = # of moles of e-s transferred = 96500 C the charge of one mole of e-s or 96,500 J/V-mol eNote that because n and are both positive values, a positive value in E leads to a negative value of G. Use the standard electrode potentials to calculate the standard free energy change, G°, for the following reaction 2Br-(aq) + F2(g) Br2(l) + 2F-(aq) 2Br-(aq) Br2(l) + 2e E°ox = -1.06 F2(g) + 2e- 2F-(aq) E°red = 2.87 2Br-(aq) + F2(g) Br2(l) + 2F-(aq) E°cell = 1.81 V G = -nE G = -(2 mol e- )(96,500 J/volt-mol e-)( 1.81 V) G° = -3.49 x 105 J = -349 kJ EMF and Equilibrium Constant “Remember in Chapter 19, we related G° to the equilibrium constant, K?” G° = -RT lnK G ° = -nE ° -nE° = -RT lnK Simplify E ° = 0.0591 logK n Calculate the K for the following reaction O2 (g) + 4H+ (aq) + 4Fe2+ (aq) 4Fe3+ (aq) + 2H2O (l) O2 (g) + 4H+ (aq) + 4e- 2H2O (l) E°red = 1.23 V 4Fe2+ (aq) 4Fe3+ (aq) + 4e- E°ox = -0.77 V O2 (g) + 4H+ (aq) + 4Fe2+ (aq) 4Fe3+ (aq) + 2H2O (l) E°cell = 0.46 V log K = n E ° = 4(0.46V) = 31.1 0.0591V 0.0591V K = 1.36 x 1031 Calculate the equilibrium constant for the reaction IO3- (aq) + 5Cu (s) + 12H+ (aq) I2 (s) + 5Cu2+ (aq) + 6H2O (l) 2IO3- (aq) + 12H+ (aq) + 10e- I2 (s) + 6H2O (l) red= 1.20V 5Cu (s) 5Cu2+ (aq) + 10e- ox= -.34V = .0591 log k .86V = .0591 log k n 10 Log K = 145.5 K = 10145.5 cell= .86V Standard Conditions G° = -RT lnK G = G° + RT lnQ Non- Standard Conditions G = G° + 2.30 RT log Q -nE = -nE ° + 2.30 RT log Q Nernst Equation When T=298K E = E ° - 2.30 RT log Q n E = E ° - 0.0591 log Q n Zn (s) + Cu2+ (aq) Zn2+ (aq) + Cu (s) E °= 1.10 V 2e- + Cu2+ (aq) Cu (s) Zn (s) Zn2+ (aq) + 2e[Zn2+] Therefore E = 1.10 ° - 0.0591 log [Cu2+] 2 If [Zn2+]=0.05M, and [Cu2+] = 0.50 M [0.05] E = 1.10 ° - 0.0591 log = 1.13V [.50] 2 Calculate the emf generated by the the following reaction Cr2O72- + 14 H + + 6I- 2Cr3+ + 3I2 + 7H2O red= 1.33V ox= -.54V [Cr2O72-] = 2.0 M [H + ] = .05 M [I-] [Cr3+] = .25 M = 1.0 x 10-5M cell= .79V = .79V - .0591 log = .68V 6 (1 x 10-5)2 (2.0)(.05)14(.25)6 Concentration Cells What happens when both cells are identical except for concentration. Nature will try to equalize the two cells. E= Eº - .0591 log Q n E = 0 - .591 log .01 = .591V .1 Lead Storage Battery anode: Pb (s) + SO42- (aq) PbSO4 (s) + 2eE °= 0.356 V cathode:PbO2(s)+ SO42-(aq)+ H+(aq)+ 2e- PbSO4(s)+ 2H2O(l) E °= 1.685 V Pb(s)+ PbO2(s)+ 4H+ +2SO4(aq) 2PbSO4(s)+ 2H2O(l) E °= 2.041 V Note that one advantage of the lead storage battery is that it can be recharged because the PbSO4 produced during discharge adheres to the electrodes Dry Cell alkaline anode: Zn (s) Zn 2+ (aq) + 2ecathode:2NH4+(aq)+2MnO2(s)+ 2e Mn2O3(s) + 2NH3(aq) + H2O (l) Fuel Cells anode: 2H2(g)+ 4OH-(aq) 4H2O(l) + 4ecathode: 4e- + O2(g) + H2O(l) 4OH-(aq) 2H2(g) + O2(g) 2H2O(l) Electrolytic Cells: Notice (+ ox.) These electrodes are inert Electrolysis voltage source acts like an electron pump (- red) Eº = ( ) Electrolysis is driven by an outside energy source Electrolysis of Aqueous Solutions Sodium cannot be prepared by electrolysis of aqueous solutions of NaCl, because water is more easily reduced than Na+: Possible Cathode reactions (Note: Not in table on AP exam) 2H2O + 2e- H2 + 2OH- E°red = -0.83 Na+ + e- Na(s) E°red = -2.71 The possible Anode reactions are: 2Cl- Cl2 2H2O 4H+ + O2 + 4e- E°ox = -1.36 E°ox = -1.23 Therefore 2Cl-(aq) Cl2(g) E°ox = -1.36 2H2O + 2e- H2 + 2OH- E°red = -0.83 E°cell > -2.19 Electrolysis of With Active Electrodes Possibilities Ni(s) Ni2+(aq) + 2e- E°ox = 0.28 2H2O 4H+ + O2 + 4e- E°ox = -1.23 anode: Ni(s) Ni2+(aq) + 2e- cathode: Ni2+(aq) + 2e- Ni(s) Electroplating creates a silver lining Quantitative Aspects of Electrolysis Calculate the mass of aluminum produced in 1.00 hr. by the electrolysis of molten AlCl3 if the current is 10.0 A. (C = amperes x seconds) (10.0A)(1.00 hr) (3600 sec)(1C) (1) (1molAl) (27.0 g Al) (1 hr) (1 A-s)(96,500C) (3) (1 mol Al) = 3.36 g of Al Quantitative Aspects of Electrolysis The half-reaction for the formation of magnesium metal upon electrolysis of molten MgCl2 is Mg2+ + 2e- Mg. Calculate the mass of magnesium formed upon passage of 60.0 A for a period of 4000 s (60.0A)(4000s)(1C)(1) (mole Mg)(24.3g) (A•s)(96,500C) (2) (mole Mg) 30.2g Mg Electrical Work since G= wmax and G = - nE then wmax= - nE The unit employed by electric utilities is kilowatt-hour (kWh =3.6 x 106J) Corrosion reactions are redox reactions in which a metal is attacked by some substance in its environment and converted to an unwanted compound. All metals except gold and platinum are thermodynamically capable of undergoing oxidation in air at room temperature The rusting of iron is known to require oxygen; iron does not rust in water unless O2 is present. E°red = 1.23 V E°red = 0.44 V Note that as the pH increases, the reduction of O2 becomes less favorable The corrosion of Iron:Protecting the surface with tin Tin has lower E°ox so less likely to oxidize until the surface is broken then it accelerates as it makes a voltaic cell with iron. Fe(s) Fe2+ + 2e- E°ox = 0.44 V Sn(s) Sn2+ + 2e- E°ox = 0.14 V The corrosion of Iron:Cathodic Protection Fe(s) Fe2+(aq) + 2e- E°ox = 0.44 V 2+(aq) + 2e- E° = 0.76V Zn(s) Zn ox Zinc is more positive and goes first and has an oxide coat that seals. sacrificial anode Galvanization The corrosion of Iron:Cathodic Protection