document
advertisement

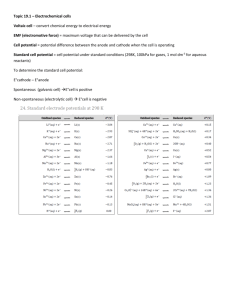
Chapter 17 Electrochemistry all based on oxidation and reduction, electrochem. puts electron movement to work! electrochemistry is the study of the interchange of chemical and electrical energy redox reactions can be split into half reactions and those reactions can be physically seperated to allow the flow of electrons to be used for energy remember that reducing agents give up electrons and oxidizing agents take electrons if force the electrons to travel along a wire to get from one half reaction to another, can use thier energy Galvanic cell- something that converts chemical energy into electrical energy Galvanic cell, labeled wire and voltometer, to allow e- to flow and measure that flow in volts salt bridge- allows ions to flow to replace electrons cathode, the place where reduction occurs metal and solution of same metal anode- place where oxidation occurs electrons flow from anode to cathode anode is oxidized cathode is reduced negative ions flow from cathode to anode to relieve neg charge buildup standard reduction potentials- table that shows the cell potential for the reduction of many metals and ions all are written as reductions, so when figuring the overall cell potential, one number will have to have the sign reversed Reduction half reaction Potential , Eo /V Li+ + e- --> Li(s)-3.045 K+ + e- --> K(s) -2.924 Ca2+ + 2e- --> Ca(s) -2.76 Na+ + e- --> Na(s) -2.7109 Al3+ + 3e- --> Al(s) -1.706 Mn2+ + 2e- --> Mn(s) -1.029 Cd(OH)2(s) + 2e- --> Cd(s) + 2OH-0.812 Zn2+ + 2e- --> Zn(s) -0.7628 Cr3+ + 3e- --> Cr(s) -0.74 Fe2+ + 2e- --> Fe(s) -0.409 PbSO4(s) + 2e- --> Pb(s) + SO42-0.356 Ni2+ + 2e- --> Ni(s) -0.23 Sn2+ + 2e- --> Sn(s) -0.1364 Pb2+ + 2e- --> Pb(s) -0.1263 2H+ + 2e- --> H2(g) 0.0000000 Sn4+ + 2e- --> Sn2+ +0.15 IO3- + 2H2O +4e- --> IO- + 4OH+0.15 SO42- + 4H+ + 2e- --> H2SO3 + H2O +0.20 Cu2+ + 2e- --> Cu(s) +0.3419 O2(g) + 2H2O(l) + 4e- --> 4OH- +0.401 IO- + H2O + 2e- --> I- + 2OH- +0.485 NiO2(s) + 2H2O + 2e- --> Ni(OH)2(s) + 2OH+0.49 I2(s) + 2e- --> 2I+0.535 Fe3+ + e- --> Fe2+ +0.770 Ag+ + e- --> Ag(s) +0.7996 ClO- + H2O(l) + 2e- --> Cl- + 2OH+0.90 NO3- + 4H+ + 3e- --> NO(g) + 2H2O(l) +0.96 Br2(l) + 2e- --> 2Br+1.065 O2(g) + 4H+ + 4e- --> 2H2O(l) +1.229 Cl2 + 2e- --> 2Cl+1.3583 MnO4- + 8H+ + 5e- --> Mn2+ + 4H2O +1.507 Au+ + e- --> Au(s) +1.68 PbO2(s) + 4H+ + SO42- + 2e- --> PbSO4(s) + 2H2O(l) F2(g) + 2e- --> 2F+2.87 +1.685 so lets take a typical reaction and see if we can figure it out! Lets look at a reaction between Iron and copper we know from last chapter that iron is more reactive, so lets write it... Fe + Cu(NO3)2 Fe(NO3)2 + Cu when we are writing these out, we often use something called line notation, it shortens up the work What gets oxidized? reduced? Fe goes to Fe +2, so that is oxidized, and Cu+2 goes to Cu, so it is reduced but to be sure we will be checking their cell potentials, Line notation would have you write Fe/Fe+2//Cu+2/Cu. oxidized first, // to show salt bridge now, how would that look on a cell? this would be iron bar in ironsomethinglike Fe(NO3)2 or FeSO4 this would be a copper bar in coppersomething like Cu(NO3)2 or CuSO4 Fe/Fe+2//Cu+2/Cu now, cell potenial, written as ξ , ξred and ξ cell oxid ξ oxid = one very common use of the energy created by these voltaic / galvanic spontaneous reactions is batteries. there are 4 basic types dry cell and alkaline dry cell lead storage nickel cadmium fuel cells dry cell and alkaline dry cell These are the batteries that run minor electronics, like AA, D, C etc the reaction is between Zn/Zn+2 and MnO2/NH+4 they run continuously, across a porous paper that acts as the salt bridge. graphite rod acts as the conductor. These are inexpensive but give little voltage alkaline dry cell uses MnO2/water, and gives a little more energy, also doesn't decay as fast, so lasts longer but they are more expensive ( still AAA, C, D, think energizer vs generic) Lead storage batteries these are rechargable, give much more power, and are therefore much more expensive and heavier. They are several alternating sheets of lead and lead dioxide, with sulfuric acid acting with it as the salt bridge. Lead is both oxidized and reduced, and the energy from your car as it runs recharges it Nickel Cadmium batteries also rechargable, these are much smaller, run things like drills, phones etc. Nickel is reduced and cadmium is oxidized have some "memory" if not completely discharged, not as cheap as dry cell Fuel cells mostly in use for rockets, this is a system that carries large quantities of fuel with it. it is the oxidation of H and reduction of O2, fueled constantly from an external source ( on the rocket) and creates water now, electrolytic cells aren't spontaneous,and don't create electricity. They use electricity ( go to powerpoint c21 4th and 7th hour)