09-Ionic Formula Lab - Goshen Local School District
advertisement

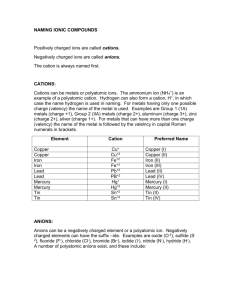
Six Bottle Problem Activity Introduction Materials Name/Number:__________________________________ Samples of six solutions are found on the counter without chemical names. It is your job to identify the cation and anion in each of the bottles so you can give the formula of each of the six unknowns. There are five different cations: ammonium, copper(II), hydrogen, iron(III), and silver(I) There are four possible anions present in the six bottles. These anions include chloride, hydroxide, nitrate, and sulfate. *pH paper Well-plate *BaCl2(aq), 0.200 M *Six unknown solutions *AgNO3(aq), 0.200 M Pre-Lab Questions Answer the following questions using complete sentences BEFORE completing the lab. 1. Define cation. Does an atom gain or lose electrons to form a cation? 2. What is a polyatomic ion? 3. Write the symbols, with charges, for the 5 possible cations. 4. Write the symbols, with charges, for the 4 possible anions. ___Check with Mrs. MacLeod Cation Determination Procedure 1. Place 5 drops of each solution in one row of the well plate, keeping track of which solution is in each well. While you are getting a sample of each solution, waft them. One solution should have a strong odor, smelling of ammonia [think cleaning products]. Record a ‘Yes’ in the smell test section for this bottle. The other solutions will be recorded as a ‘No.’ This solution contains the cation ammonium. 2. Examine each of the six solutions and record the color of each in your data table. The colors will allow you to determine several cations in the bottles: Blue = copper(II) Orange = iron(III) 3. Using both a red and blue piece of litmus paper, test the pH of each solution. Record whether the red litmus paper turned blue or had no change [record blue or NC, for no change], and whether the blue litmus paper turned red or had no change [record red or NC]. Solutions which caused the blue pH paper to turn red [and do not already have a cation identified!] will contain the hydrogen ion. 4. You should have identified the cation in 5 of the bottles. There is only one remaining cation for the last bottle. Anion Determination Procedure 5. Using the information learned from the pH test, you can identify which solutions contain hydroxide ions. These will have turned the red pH paper blue. 6. Add a drop of the solution containing silver to each of the remaining solutions with unknown anions in the well plate from the pH test. Silver is known to react with chloride ions to form a solid (called a precipitate, which will look cloudy). Record in your data table the solutions turned cloudy or remained clear. Any solution that you do not test with the silver should be recorded as N/A. 7. Now place 5 new drops of only the solutions that still have unknown anions in a new row on the well plate. Add a drop of the barium solution to these final unknowns. Barium is known to react with sulfate ions to form a precipitate. Record which solutions turned cloudy or remained clear; any solution that you do not test with the barium should be recorded as N/A. 8. The remaining solutions will not cause any reaction, no matter what you add to them. These will all contain the last remaining possible anion, nitrate. Clean-up 9. Rinse the well plate thoroughly with water, using a test tube brush as needed in any well. Return your station to its previous state, put goggles away, wash your hands, and check out with the instructor. Data/Observations Bottle Smell Color Red Litmus Blue Litmus Silver Test Barium Test Cation Anion 1 2 3 4 5 6 Questions 1. Write the correct formula for the compound in each bottle. Be sure to include the number for each solution. 2. Name the compound in each bottle, including roman numerals when appropriate. Again, label the number for each solution.