Biochemistry Biological Molecules Carbohydrates
advertisement

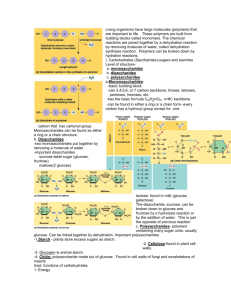
Biochemistry Biological Molecules Carbohydrates Monosaccharides Elements: carbon (C), hydrogen (H) and oxygen (O). The ratio of H to O is always 2 : 1. Monosaccharides are called simple sugars because they are the simple subunits joined together to make larger carbohydrates - and they taste sweet. The general formula of a monosaccharide is a pentose, if n = 6 the sugar is called a hexose. . If n = 5 then the sugar is called Examples Pentoses : ribose and deoxyribose found in DNA and RNA. Hexoses : glucose, galactose and fructose. Glucose is our major fuel. It is the sugar which is transported in our bloodstream to our cells where it is broken down during respiration to release energy. Dissaccharides A disaccharide is made of two monosaccharides joined together by a glycosidic bond. The molecular formula for disaccharides is C12H22O11. The monosaccharide components of each of the disaccharides are: sucrose = glucose + fructose lactose = glucose + galactose maltose = glucose + glucose If sucrose is given the value of one 'spoonful' for sweetness, then this is how other natural sugars, and some commercial sweeteners, compare: . One spoon of substance Equivalent to spoons of sucrose Fructose 1.7 Glucose 0.7 Maltose/galactose 0.3 Lactose 0.2 Aspartame 180 (artificial sweetner in fizzy drinks and tabletop sweeteners) Saccharin 400 (artificial sweetner in fizzy drinks and tabletop sweeteners) Polysaccharides Polysaccharides are large carbohydrate molecules made up of many monosaccharides held together by glycosidic bonds. Starch, made up of glucose, is shown below. Starch Cellulose Two molecules make up starch: Long unbranching chains which lie parallel to each other and are connected by hydrogen bonds forming cross links between the chains. Glycogen Monomer Molecular structure Highly branched, tree-like Amylopectin (branched) Amylose (coiled not branched) Found in Function The chains are layered into bundles which are laid down at right angles to each other in the cell wall Plants only: all plant cell walls Animals Plants only, found as only, found starch granules in as tiny photosynthetic cells in granules in particular muscle and liver cells Storage form Energy reserve - found One of the main of glucose in in storage organs, e.g. components of the cell muscles and potato wall liver hydrolysis of the glycogen releases glucose when blood glucose levels drop Roles of Carbohydrates Monosaccharides, glucose in particular, are an important source of energy for cells. Ribose and deoxyribose sugars make up part of RNA and DNA. The soluble monosaccharides are essential for the transport of food around animals (glucose). Mammalian milk contains 5% lactose, so it's a major source of carbohydrates for babies. Glycogen and starch are important energy reserves for animals. Both are insoluble in water which means they remain inside cells. Glycogen and starch are compact structures. Branched molecules can take up less space in a cell but are made of more glucose monomers. This means more energy is stored in less space! The cellulose in cell walls has a high tensile strength - this means it is tough. But it is permeable to water and most substances can pass straight through it. Proteins Structure of proteins The basic structure of a protein is a polypeptide chain. Many amino acids (from as few as forty, to a few hundred) join together by means of peptide bonds in a specific sequence (order) to form a polypeptide chain. There are 20 different amino acids found in proteins, yet over tens of thousands of different possible proteins. This variety is possible because there are over 8 000 ways that 20 amino acids can be arranged in a tripeptide, i.e. just 3 amino acids. Enzymes speed up chemical reactions, e.g. salivary amylase hydrolyses starch to maltose. Proteins are essential constituents of many parts of the body, e.g. hair, nails, skin, bones, tendons, cartilage and ligaments. Some globular proteins are antibodies which bind to foreign microbes. Other globular proteins are found in plasma membranes - they aid the transport of large molecules into and out of cells. Haemoglobin and myoglobin are transport proteins - carrying oxygen in the blood and muscles respectively. Some hormones are proteins, e.g. insulin, which controls blood sugar levels. Proteins are amphoteric: they can act either as an acid or a base. So they make good buffers - resisting changes in pH, e.g. in tissue fluids, proteins keep pH levels steady (an important aspect of homeostasis). Lipids a mixed group of organic compounds are grouped together because they have a high proportion of CH2 are, as a result, not soluble in water but are soluble in non-polar (organic) solvents, e.g. ether or chloroform divided into several groups: o fats, oils and waxes o steroids o phospholipids Fats, oils and waxes Fats and oils are composed of C, H and O, as in carbohydrates. But the ratio of H : O is higher in fats (i.e. more H atoms per O atom in fats than in carbohydrates). The main difference between fats, oils and waxes is their melting points. Monomers are alcohols (glycerol) and fatty acids. A condensation reaction produces a triglyceride and water molecule when glycerol and three fatty acids react together. An unsaturated fatty acid contains one or more carbon double bonds. There is potential for more hydrogen atoms to become part of the tail structure if the carbon double bond were to be broken. A saturated fatty acid is 'filled' with hydrogen atoms - every available carbon bond of the central chain is taken up by hydrogen atoms. Fats (triglycerides) in the diet are classified either as saturated or unsaturated. A saturated fat is formed when saturated fatty acids react with glycerol. An unsaturated fat is made when unsaturated fatty acids react with glycerol. Along with proteins, phospholipids are essential components of all cell membranes. A phospholipid is composed of two fatty acids, a glycerol and a phosphate group, The cell membrane is composed of a bilayer of phospholipids. The hydrophobic tails face each other (pointing inwards) and the hydrophilic heads point outward and are in contact with the aqueous external and internal cellular environments