File
advertisement

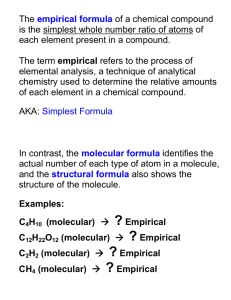
March 6, 2015 Pick up turned back docs 2. Pick Up Calculator 1. Warm up Using the Sticky Note on your desk answer the following: For the Unit Mole Conversions 1. I am strongest at ___________________ 2. The question I still have is ________________________ Homework 3-6 Finish Empirical and Molecular Handout Important Dates Monday 3-2 – Smore Chemistry Tuesday 3-3 – ACT (homeroom) Wednesday 3-4 – Demo / Reaction Stoich Thursday 3-5 – Quiz / #TBT Friday 3-6 – Composition Stoich / Mole conversion assessment Today is March 6, 2014 Topic: Reaction Stoichiometry Lesson Objectives: SWBAT achieve 85% or higher on their mole conversion assessment Essential Question: 2.2 Analyze chemical reactions in terms of quantities, product formation, and energy. Agenda 3-6 Do Now 2 Practice Problems Assessment Composition Stoich Guided Notes HW Headstart Mole Conversion Review How many grams are in 6 moles of Iron? How many atoms are in 45 grams of water ? Test Today is March 6, 2014 Topic: Composition Stoichiometry Lesson Objectives: SWBAT to determine the composition of a substance using multiple composition stoich methods Essential Question: 2.2 Analyze chemical reactions in terms of quantities, product formation, and energy. Calculating Percentage Composition Calculate the percentage composition of magnesium carbonate, MgCO3. Formula mass of magnesium carbonate: 24.31 g + 12.01 g + 3(16.00 g) = 84.32 g 24.31 Mg 100 28.83% 84.32 12.01 C 100 14.24% 84.32 48.00 O 100 56.93% 84.32 100.00 Practice 1. 2. 3. H2O (calculate O) CaCOOH (Calculate Ca) NaOH (Calculate H) Empirical Formula The formula that gives the simplest whole-number RATIO of the atoms of each element in a compound “Simplified” Empirical Formula Chemical Formula (Molecular Formula) H 2 O2 C6H12O6 CH3O C2H4O2 Empirical Formula HO Determining Empirical Formulas Steps: 1. Find mole amounts 2. Divide each mole by the smallest mole 3. Multiply until all whole numbers 1. Percent to mass 2.Mass to Mole 3.Divide by small 4.Multiply ‘till whole Determine the empirical formula for a compound containing 2.128g Cl and 1.203g Ca Molecular Formula The formula that gives the ACTUAL NUMBER of atoms of each element in a compound “Real Life” Determining Molecular Formula Steps: 1. Find the empirical formula 2. Calculate the empirical formula mass or “EFM” (think molar mass) 3. Divide the molar mass by the “EFM” (this gives you the “factor”) 4. Multiply empirical formula by the factor Find the molecular formula for a compound who molar mass is ~124.06 and empirical formula is CH2O3