Carboxylic Acid
advertisement

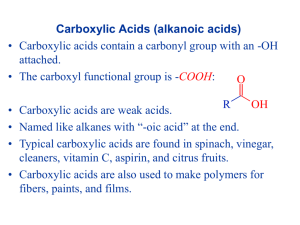
Chapter Sixteen Carboxylic Acids, Esters, and Other Acid Derivatives Carboxylic Acids Fig. 16.1 The three simplest carboxylic acids: methanoic acid, ethanoic acid, and propanoic acid. Chapter 16 | Slide 2 of 39 Carboxylic Acids → Fig. 16.2 Benzoic acid molecule Chapter 16 | Slide 3 of 39 Carboxylic Acids Carboxylic acids contain the carboxyl group on carbon 1. • A carboxyl group is a carbonyl attached to a hydroxyl • Acts differently than an alcohol or a carbonyl compound O CH3 — C—OH = CH3—COOH carboxyl group Chapter 16 | Slide 4 of 39 Naming Carboxylic Acids Formula IUPAC alkan -oic acid Common prefix – ic acid HCOOH methanoic acid formic acid CH3COOH ethanoic acid acetic acid CH3CH2COOH propanoic acid propionic acid CH3CH2CH2COOH butanoic acid butyric acid Chapter 16 | Slide 5 of 39 Naming Rules • Identify longest chain containing the carboxyl group • (IUPAC) Number carboxyl carbon as 1 • (Common) Assign , , g to carbon atoms adjacent to carboxyl carbon CH3 | CH3 — CH—CH2 —COOH IUPAC 3-methylbutanoic acid Common -methylbutryic acid Chapter 16 | Slide 6 of 39 Aromatic Carboxylic Acids • Benzoic Acid is the aromatic carboxylic acid • Locates substituents by assigning 1 to the carbon with the carboxyl group O OH C O OH O C OH C Cl Benzoic acid 3-chlorobenzoic acid CH3 4-methylbenzoic acid Chapter 16 | Slide 7 of 39 Chapter 16 | Slide 8 of 39 Chapter 16 | Slide 9 of 39 Properties of Carboxylic Acids • Like alcohols, carboxylic acids form strong intermolecular hydrogen bonds. • Most carboxylic acids exist as dimers. – Boiling points higher than alkanes of similar MW. – Small carboxylic acids (1-4 carbons) are soluble in water O H O H3C CH 3 O H O Chapter 16 | Slide 10 of 39 Chapter 16 | Slide 11 of 39 Synthesis of Carboxylic Acids • Synthesized from aromatic rings, primary alcohols and aldehydes. R Oxidizing agent R OH Carboxylic Acid O R Chapter 16 | Slide 12 of 39 Oxidation of Aromatic Compounds • Benzene does not react with KMnO4. • Alkyl groups on the ring (-R) are readily oxidized though. • One product for all reactions. O R OH + KMnO4 Chapter 16 | Slide 13 of 39 Oxidation of Aromatic Compounds with KMnO4 O OH Chapter 16 | Slide 14 of 39 • Oxidation of primary alcohols and aldehydes: O OH CrO3, H3O+ or Na2Cr2O7, H2O, CH3CO2H R O R R OH O H AgNO3 NH4OH R OH Chapter 16 | Slide 15 of 39 • Oxidation of alcohols to carboxylic acids. O OH CrO3, H3O+ HO CrO3, H3O+ OH OH O Chapter 16 | Slide 16 of 39 Properties • Carboxylic acids are weak acids – Stronger acids than alcohols CH3COOH + H2O • Neutralized by a base CH3COOH + NaOH CH3COO– + H3O+ CH3COO– Na+ + H2O Chapter 16 | Slide 17 of 39 • Carboxylic acids are obviously acidic. • Stronger acids than alcohols, but weaker than mineral acids. • Will react with NaOH to give metal carboxylates. • There is a large range of acidities depending on the substituents on a carboxylic acid. O O + NaOH R OH + H2O R O-Na + Chapter 16 | Slide 18 of 39 Carboxylate Ions • The conjugate base of a carboxylic acid – Formed when a carboxylic acid loses a proton – Named by dropping the -ic acid ending and replacing it with –ate • CH3CH2COO• CH3COO• CH3CH2CH2COO- Propionate Ethanoate (Acetate) Butanoate Chapter 16 | Slide 19 of 39 Carboxylate Salts • An ionic compound in which the negative ion is a carboxylate ion – Naming: the positive ion is named first, followed by a separate word giving the name of the negative ion – Converted back to a carboxylic acid by the addition of a strong acid • CH3CH2COO-Na+ • CH3COO-K+ • CH3CH2CH2COO-Li+ Sodium Propionate Potassium Ethanoate Lithium Butanoate Chapter 16 | Slide 20 of 39 Carboxylic Acids Table 16.3 Chapter 16 | Slide 21 of 39 Esters ← Fig. 16.12 Methyl and ethyl esters of acetic acid. Chapter 16 | Slide 22 of 39 Ester In an ester, the H in the carboxyl group is replaced with an alkyl group O CH3 — C—O —CH3 = CH3—COO —CH3 ester group Sulfur analogs of esters are called thioesters (sulfur replaces the hydroxyl oxygen) Chapter 16 | Slide 23 of 39 Naming Esters • Name the alkyl from the alcohol –O• Name the acid with the C=O with –ate acid alcohol O methyl CH3 — C—O —CH3 Ethanoate (acetate) methyl ethanoate (IUPAC) methyl acetate (common) Chapter 16 | Slide 24 of 39 Chapter 16 | Slide 25 of 39 Esters Chapter 16 | Slide 26 of 39 Esterification: Preparation of Esters • Reaction of a carboxylic acid and alcohol • Acid catalyst O CH3 — C—OH + HO—CH2CH3 H+ O CH3 — C—O—CH2CH3 + H2O Chapter 16 | Slide 27 of 39 Hydrolysis: Breaking Up Esters • Esters react with water and acid catalyst • Split into carboxylic acid and alcohol O H+ H — C—O—CH2CH3 + H2O O H — C—OH + HO—CH2CH3 Chapter 16 | Slide 28 of 39 Hydrolysis Chapter 16 | Slide 29 of 39 Saponification • Esters react with bases • Produce the salt of the carboxylic acid and alcohol O H 2O CH3C—OCH2CH3 + NaOH O CH3C—O– Na+ + HOCH2CH3 salt of carboxylic acid • Saponification reactions produce soaps Chapter 16 | Slide 30 of 39 Soaps • Carboxylate salts Chapter 16 | Slide 31 of 39 • The following figure represents soap. • Which end of the anion is hydrophobic? Chapter 16 | Slide 32 of 39 • How does soap interact with grease? Chapter 16 | Slide 33 of 39 Chapter 16 | Slide 34 of 39 Chapter 16 | Slide 35 of 39 Chapter 16 | Slide 36 of 39 Dicarboxylic Acids Chapter 16 | Slide 37 of 39 The C2 dicarboxylic acid, oxalic acid, contributes to the tart taste of rhubarb stalks. Chapter 16 | Slide 38 of 39 Polyfunctional Carboxylic Acids • Carboxylic acids that contain one or more additional functional groups – Unsaturated acids • Contain a multiple bond – Hydroxy acids • Contains an additional hydroxyl group – Keto acids • Contains an additional carbonyl group • Many monoacids and polyfunctional carboxylic acids are intermediates in metabolic reactions Chapter 16 | Slide 39 of 39