Macromolecules Chart: Structure and Components
advertisement
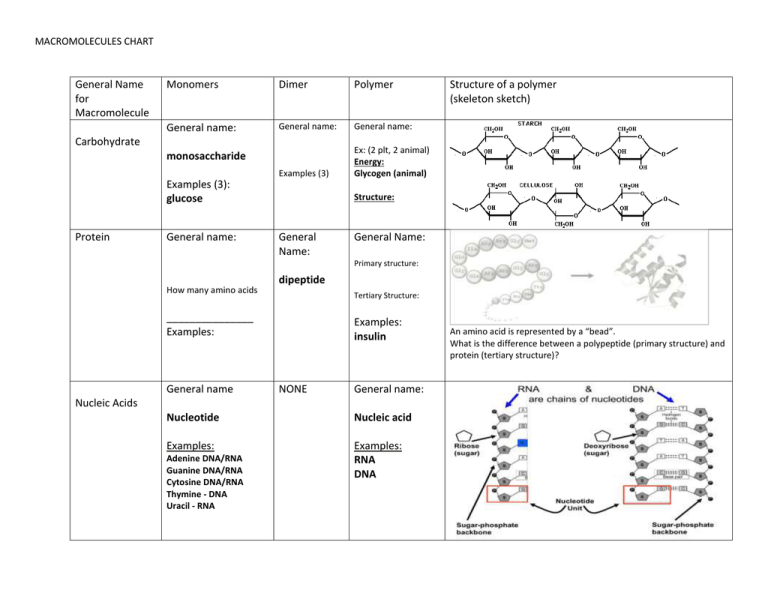
MACROMOLECULES CHART General Name for Macromolecule Monomers Dimer Polymer General name: General name: General name: Examples (3) Ex: (2 plt, 2 animal) Energy: Glycogen (animal) Carbohydrate monosaccharide Examples (3): glucose Protein General name: Structure of a polymer (skeleton sketch) Structure: General Name: General Name: Primary structure: How many amino acids dipeptide Tertiary Structure: _______________ Examples: General name Examples: insulin NONE General name: Nucleic Acids Nucleotide Nucleic acid Examples: Examples: RNA DNA Adenine DNA/RNA Guanine DNA/RNA Cytosine DNA/RNA Thymine - DNA Uracil - RNA An amino acid is represented by a “bead”. What is the difference between a polypeptide (primary structure) and protein (tertiary structure)? MACROMOLECULES CHART 1. Show dehydration synthesis between two carbohydrate monomers The diagrams below indicated HOW you will need to write these Show DS between two amino acids + + H2O If you are given a single monosaccharide, you should be able to create a second monosaccharide, and then show how bonding takes place, the number of water molecules formed and the structure of the polysaccharide. You only need to use structures that are hexagons contain the hydroxyl groups that will be bonding If you are given one amino acid (with an R group) you should be able to draw a second amino acid oriented the correct way, show which atoms will form water (OH on COOH and H on NH2), and show the di or polypeptide with the correct number of water molecules. . Show hydrolysis of a polysaccharide (made of three monomers). Show hydrolysis of 3 amino acids. Give a polymer of either amino acids or polysaccharides you should be able to show how they split into monomers, and be able to determine the number of monomers formed, # of water molecules needed for hydrolysis and be able to draw the structure at the detai level shown in the DS example. MACROMOLECULES CHART LIPIDS (NO MONOMERS OR POLYMERS) http://www.wisc-online.com/Objects/ViewObject.aspx?ID=AP13204 Definition: Lipids are molecules that are hydrophobic (insoluble in water). WHY? non-polar covalent bonds between C and H aren’t attracted to the polar bonds of water. Four categories of lipids: tryglycerides (fats and oils), phospholipids, waxes and steroids (on the back). Type of Lipid Overall Structure Components of Lipid Examples Triglycerides (Neutral Lipids) Glycerol (fats and oils) What is this structure called? What do the bends represent? Phospholipids Similar to triglycerides, but only TWO fatty acids and The glycerol has a phosphate attached (the phosphate part of the molecule is charged (and can dissolve in water, the CH tails cannot! Fatty acids A triglyceride can be a fat or an oil. Triglycerides contain a glycerol with 3 fatty acids (2 FA are shown) UNSATURATED VS. SATURATED Saturated NO double bonds between carbons. Usually in animals Where do I find phospholipids? In the membranes of cells RECOGNITION ONLY Head (hydrophilic) and Tail (hydrophobic) MORE TYPES OF LIPIDS (see the back) Unsaturated One or more double bond between carbons in the FA Usually in plants (oils) MACROMOLECULES CHART What is a steroid? Steroids include cholesterol, progesterone, testosterone, cortisol Steroids Don’t worry about structure. FYI only What is one wax tat is found in all living things? Waxes Don’t worry about structure. Waxes are a combination of an alcohol and a fatty acid For all macromolecules, you should be able to provide examples and uses for NATURAL versions of these organic molecules in living things (NOT food). Fats