LIPIDS Student handout Weir
advertisement

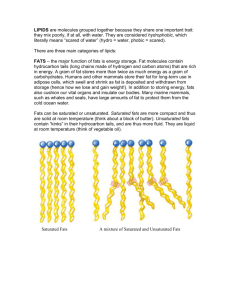
HS 20 - Weir LIPIDS What is a Lipid? Lipids are a diverse group of organic compounds that are _________________ in water. Lipids are present everywhere! They are the reason that you are unable to wash your face with water alone; you need some type of soap to break the insoluble lipids before you can wash them away. Lipids can serve as key components in _____________________________, act as cushions for delicate organs of the body, serve as carriers of vitamins, and _______________________________and other important chemicals. Lipids are __________________________________ This means that they resist dissolving in water, but can dissolve in other non-polar molecules Most lipids are composed of two main structural components 1) Glycerol = ____________________________________________________________ 2) Fatty acid chain = ______________________________________________________ ____________________________________________________________________ Fatty acids are the ________________________of the fat in our bodies and in the food we eat. During digestion, the body ______________________________ into fatty acids, which can then be absorbed into the blood. Fatty acid molecules are usually joined together in groups of three, forming a molecule called a triglyceride. Fatty acids have many important functions in the body, including________________________. If glucose (a type of sugar) isn't available for energy, the body uses _____________________________________________________________________________. Lipids include molecules like: HS 20 - Weir We will study 4 types of Lipids: 1) Triglycerides 2) Waxes 3) Phospholipids 4) Steroids 1) Triglycerides Formed by _____________and ____________________________ ________ of the fats we eat are Triglycerides Tri = 3 fatty acid chains, Glyceride = glycerol backbone Triglycerides that are ____________ at room temperature (ex. Butter) are called ______ o Can also be called _______________________________ Saturated Fats: Contain __________ single bonds between carbons These can be found in: ________________________________________________________________________ __________________________________________________________________________ * Called saturated because they are saturated with more Hydrogen * ______________________________ are usually saturated fats. Triglycerides that are ____________at room temperature (ex. Olive oil) are called _____ o Can also be called _____________________________ HS 20 - Weir Unsaturated Fats: Contain ______________________________________ These can be found in: ________________________________________________________________________ ___________________________________________________________________________ **Since there is a double bond there is _________ room for hydrogen = unsaturated with hydrogen Saturated Unsaturated Structure State at Room Temperature Melting Point Cholesterol Recommended Consumption Trans Fat: An unhealthy substance, also known as trans fatty acid, made through the chemical process of hydrogenation of oils. Hydrogenation solidifies liquid oils and increases the ____________________ and the flavor stability of oils and foods that contain them. Trans fat is found in vegetable shortenings and in some margarines, crackers, cookies, snack foods and other food Trans fats are also found in abundance in "french fries." To make vegetable oils suitable for deep frying, the oils are subjected to hydrogenation, which creates trans fats. Among the hazards of fast food, "fries" are prime in purveying trans fats. HS 20 - Weir 2)Waxes Are long chains of fatty acids that are tightly packed and linked to alcohols or carbon rings They have a _______________________________ They _____________ water In the human body, waxy secretions are used for ____________________, and lubrication of the skin and hair 3)Phospholipids Composed of: ◦ A glycerol backbone ◦ ______________________________________________ ◦ A phosphate group with choline as the “head” The ________ of the phospholipid has a negative and positive charge. This makes it _______________. Polar molecules can dissolve in water (since water is also polar) We call this _______________________. The tails of the phospholipid are non-polar. This means they_______________ like water. They will not dissolve in water. We call this ________________________. This gives phospholipids a very unique structure. They have one end that is hydrophilic and one end that is hydrophobic Because of this unique characteristic. Phopholipids arrange themselves in a specific way called the ________________________________________ This bilayer makes up the membranes of all the cells in the ____________________. HS 20 - Weir 4) Steroids Steroids are hydrophobic and insoluble in water Steroids are naturally produced in the body Composed of: ________________________________________ Examples of Steroids:______________________________________________________ Fats and Diet Fats are a required part of our diet although problems arise when you consume too much fat. Doctors recommend no more than _______ of total energy intake should be in the form of fats. ____________________________ has been associated with diets high in saturated fats. _______________________________ and obesity have also been linked to breast cancer, colon cancer and prostate cancer. Obesity has also been linked to high blood pressure and _________________________. What is Cholesterol? Cholesterol is a type of ________ found in your blood. Your ____________ makes cholesterol for your body. You also can get cholesterol from the______________________________. Meat, fish, eggs, butter, cheese, and whole or low-fat milk all have cholesterol in them HS 20 - Weir TEXTBOOK QUESTIONS In the textbook read page 64 and answer the following questions: 1) In what ways do plant and animal fats differ? (This questions is from page 62) 2) Heart disease is the number one killer of North Americans. What can cause it? 3) List a few items that may contribute to reducing blood cholesterol levels? 4) What are the 2 types of cholesterol “packaging”? Which one is good and which one is bad? 5) Define plaque. 6) Evidence shows that what 2 things in a diet reduce cholesterol?