CHEMISTRY A PRACTICE PROBLEMS For all problems you must
advertisement

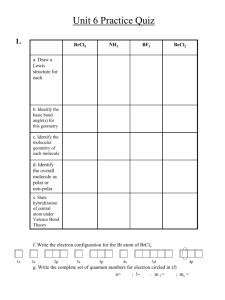
CHEMISTRY A PRACTICE PROBLEMS For all problems you must show all of your work and circle your answers. 1. Convert 74.5 ml to Liters. 2. Convert 2398 mg to kilograms. 3. Use 1 inch = 2.54 cm to convert 200.0 kilometers to miles. 4. Use 1 kg = 2.2 pounds to convert Mr. Portis' weight (175 pounds) into grams. 5. The Kentucky Derby race is 1.25 miles. Determine the length of the race in rods. 5 ½ yards = 1 rod 40 rods = 1 furlong 8 furlongs = 1 mile Determine the number of significant figures in: 6. a) 37.90 cm 7. a) 600.020 meters 25 b) 240 pounds b) 7.1010 x 10 miles c) 0.000932 mg c) 0.8070 grams 8. ADD. 97.42 g + 100.3 g + 6.206 g + 0.058 g 9. MULTIPLY. 12.45 m x 6.21 m x 9.804 m Convert each temperature into the other two units. Nearest degree is fine; but you must show all of your work. o 11. -114 C o 12. 295 F 13. 215 K 3 3 14. A sample of uranium has a density of 18.9 g/cm and a volume of 7531.64 cm . Determine its mass. 3 15. A metallic sample has a mass of 292.1 g and a volume of 14.45 cm . Determine the density of this metal. 16. Methyl alcohol has a density of 0.798 g/ml. Determine the volume of a sample that has a mass of 143.82 g. 3 17. According to the Handbook of Chemistry and Physics; the density of aluminum is 2.70 g/cm . 3 In the lab, you determine the density to be 2.55 g/cm . Determine your: a) absolute error b) percent error o 18. According to the Handbook of Chemistry and Physics; the melting point of cholesterol is 148.5 C. o In the lab, you determine it to be 141.1 C. Determine your: a) absolute error b) percent error o 19-20. You design an experiment to measure the speed of an oxygen molecule at 20 C. Measured Values 411 cm/sec 406 cm/sec 404 cm/sec Accepted Value Determine your: 440 cm/sec AVERAGE FINAL REPORTED VALUE DEGREE OF UNCERTAINTY ABSOLUTE ERROR OBSERVATIONAL ERROR PERCENT ERROR 21-22. You design an experiment to measure the atomic radius of the -4 carbon ion using X-ray crystallography. Measured Values 2.22 A 2.75 A 2.92 A 2.10 A Accepted Value Determine your: 2.60 A AVERAGE FINAL REPORTED VALUE DEGREE OF UNCERTAINTY ABSOLUTE ERROR OBSERVATIONAL ERROR PERCENT ERROR 23. a) In which of the previous 2 experiments was your accuracy better? Explain your answer. b) In which of the previous 2 experiments was your precision better? Explain your answer. o o 24. A 15.32 g sample of copper wire is heated from 22 C to 128 C. To do so it must absorb 624.49 Joules. Determine the specific heat of copper. o o 25. A 15.32 g sample of gold wire is heated from 23 C to 128 C. To do so it must absorb 205.9 Joules. Determine the specific heat of gold. o 26. The specific heat of silver is 0.236 J/g C. A sample of silver absorbs 1619.5 Joules of heat as its o o temperature rises from 50.0 C to 85.0 C. Determine the mass of the silver sample. o 27. The specific heat of tungsten wire is also 0.1325 J/g C. As a filament in a light bulb it may absorb o 66.919 Joules for a 1.11 g sample. If the initial temperature of the tungsten is 23.0 C; determine the final temperature of the tungsten filament sample. o 28. The specific heat of nickel wire is 0.4435 J/g C. A 2.11 g sample of nickel wire loses o 70.18 Joules of heat. If the initial temperature of the nickel is 143.0 C; determine the final temperature of the nickel sample. o 29. It is necessary to take 145.0 g of solid ethyl alcohol at its melting point (-114 C) and produce o 145.0 g of gaseous ethyl alcohol at its boiling point (78 C). Determine the total amount of heat this would require. For ethyl alcohol: heat of vaporization: 2260 J/g heat of fusion: 109 J/g o specific heat: 2.30 J/g C o 30. For water: SH = 4.184 J/g C; Δ HFus = 335 J/g; Δ HVap = 2330 J/g. o Determine the amount of heat a 25.0 g sample of water must lose; if it starts as steam at 100 C o and changes to a block of ice at 0 . Complete the following chart. The first 8 isotopes are neutral. SYMBOL Tc CHARGE 99 NUMBER OF PROTONS NUMBER OF ELECTRONS NUMBER OF NEUTRONS . 0 43 . 212 83 Bi 0 . 0 54 81 . 0 93 148 . 195 0 117 . Pb 0 128 . 193 0 117 . 253 Cm 0 . -3 33 44 . +2 38 52 . Te 130 -2 . 1. Chemistry scholars are known for their quantitative abilities. Instead of chanting "Go Blue! ; Go Gold!" at basketball games; they would, of course, prefer that the students chant the wavelengths of these colors (in nanometers). Please fill in the blank with the appropriate wavelength. 14 14 (the frequency for blue is 6.65 x 10 Hz ; the frequency for gold is 5.22 x 10 Hz) Go ________________________________! ; Go ________________________________! 8 2. FM Radio station WKFR has a frequency of 103.3. This means that the frequency is 1.033 x 10 Hz for the radio waves they transmit. a) Determine the wavelength of its radiowaves in miles. b) Determine the wavelength of its radiowaves in meters. c) Now determine the energy of the radiowaves. 3. The last thing Dr. Bruce Banner noticed before he turned green and started growing out of his clothes; was the energy indicator on the light meter in his lab. The energy indicator was locked on -12 1.55 x 10 Joules. a) Determine the frequency of the radiation that was saturating his lab. b) Determine the wavelength (in meters) of the radiation that was saturating his lab. 4. Highway flares give off an intense red light with a wavelength of 640 nm. It is caused by the brightline spectrum of strontium. Determine the change in energy required to produce this light. 5. The data coming back to Earth from the Cassini spacecraft orbiting Jupiter by radio wave takes 35.00 3 minutes (2.100 x 10 seconds) to reach the Earth. How far (in miles) from Earth must the Cassini spacecraft be? 6. Calculate the de Broglie wavelength for an alpha particle (mass = 6.696 x 10 7 to a speed of 8.54 x 10 m/sec. -27 kg) that is accelerated Remember to show the set-up of your problem and circle your answers. 7. Calculate the Atomic Weight for Dysprosium to three decimal places. (Based on the following data.) Isotope Dy-156 Dy-158 Dy-160 Dy-161 Dy-162 Dy-163 Dy-164 Atomic Mass 155.9238 amu 157.9240 amu 159.9248 amu 160.9266 amu 161.9265 amu 162.9248 amu 163.9288 amu Percent Abundance 0.052% 0.090% 2.29 % 18.88 % 25.53 % 24.97 % 28.18 % 8. Determine the Percent Abundance (to three decimal places) for each of the isotopes of Vanadium. Atomic Weight = 50.942 amu Isotope Vanadium-50 Vanadium-51 Atomic Mass 49.9472 amu 50.9440 amu Questions 9-56: are worth one point each. Indicate the atomic number and the atomic symbol of the element(s) that best fit each description. 9. An Alkali Metal with 2 electron shells. 10. A Halogen with 4 energy levels. 11. A member of the Carbon Family with 3 energy levels. 12. A Noble Gas with the sixth shell as its valence shell. 13. An Alkali Earth Metal with the fourth shell as its last shell. 14. An element with 3 valence electrons and 4 energy levels. 15. An element with 4 valence electrons and 3 electron shells. 16. An element that forms a +1 ion and has three energy levels. 17. An element that may form a +3 ion and has two energy levels. 18. A nonmetal element that forms a -3 ion and has the third shell as its valence shell. 19. An element that forms a -2 ion and has three energy levels. 20. The metalloid member(s) of the Nitrogen family. 21. The element in the third period and in the Oxygen Family. 22. The element in the fourth period and Alkali Metal Family. 23. The element in the fifth period and the Boron Family. 24. The main group element in the sixth series and has a +4 ion charge. 25. The Noble Gas that has five electron shells. 26. The element in the third series and has a -1 ion charge. 27. The member of the Alkali Earth Metal Family listed last alphabetically. 28. The member of the Oxygen Family that is a yellow powder and helps make rubber less brittle. 29. The member of the Boron Family that is an exception to the metalloid rule. 30. A Transition Metal with four electron shells and that has a name that would be listed last alphabetically. 31. A member(s) of the Lanthanide Series that begins with the letter "N". 32. A member of the Actinide Series named after a country. 33. A member of the Nitrogen Family that is a toxic, steel-gray metalloid. 34. The member of Family VIIIA that is used as a lifting gas. 35. The most abundant element on earth. 36. The most abundant element in the universe. 37. The most abundant metal in the body 38. The member of the Halogen Family that you have seen sublimate. 39. The only liquid (Transition) Metal at room temperature. 40. The largest atom in the second period. 41. The smallest atom in the third series. 42. The smallest atom in the Alkali Metal Family. 43. The largest atom in the Boron Family. 44. The atom in the third period with the largest IE. 45. The atom in the fourth series with the lowest IE. 46. The element in the third period with the highest EA. 47. The element in the second period with the lowest EA. 48. The member of the Actinide Series that begins with the letter "T". 49. The member of the Boron Family with the lowest EN. 50. The member of the third period with the highest EN. 51. The more active metal: Rubidium or Titanium. 52. The more active nonmetal: Sulfur or Phosphorus. Indicate the element that has an electron configuration ending: 1 1 53. 4 px py pz 1 2 2 2 2 2 54. 6 s 5 d1 d2 d3 d4 d5 (the 4f's are also full) 2 2 1 1 1 1 1 55. 4 f1 f2 f3 f4 f5 f6 f7 FIRST IONIZATION ELEMENT ENERGY IN kJ/mole 2 1 1 1 56. 4 s 3 d1 d2 d3 d4 d5 SECOND IONIZATION ELECTRON AFFINITY ENERGY IN kJ/mole IN kJ/mole . Potassium 100 729 11.6 . Calcium 141 274 -37.4 . Bromine 272 384 77.6 . Questions 57-63: are worth three points each. 57. Write a complete and balanced equation for the formation of the positive one Potassium ion. 58. Write a complete and balanced equation for the formation of the negative one Bromide ion. 59. Write a complete and balanced equation for the formation of the Calcium (+2) ion. 60. Write a complete and balanced equation for the formation of the Calcium (-1) ion. Draw full and complete Bohr Atomic Models for the following elements. Two Points Each. 1. Niobium 2. Americium Write full and complete Electron Configurations for the following elements. Two Points Each. 3. Germanium 4. Bismuth 5. Chromium 6. Tin Write full and complete Orbital Notations for the following elements. Two Points each. 7. Nitrogen 8. Molybdenum 9. Silicon 10. Magnesium Write Abbreviated Electron Configurations for the following elements. Two Points Each. 11. Rubidium 12. Silver 13. Nobelium 14. Actinium Write Abbreviated Orbital Notations for the following elements. Two Points Each. 15. Vanadium 16. Protactinium 17. Lanthanum 18. Barium For Problems 19-24: Give the set of quantum numbers that indicate the electron that is described. Two Points Each. 19. the electron is located in the third f orbital of the fourth shell and spins against the magnetic field 20. the electron is located in the px orbital of the third shell and spins with the magnetic field 21. the first electron placed in the third d orbital of the fifth energy level 22. the second electron placed in the s orbital of the sixth energy level 23. the last electron that is placed in the electron configuration of Arsenic 24. the last electron that is placed in the electron configuration of Iron For Problems 25-30: Completely describe the electrons indicated by each set of quantum numbers. n l ml s 25. 4 2 +1 -1/2 26. 2 1 0 +1/2 27. 6 0 0 -1/2 28. 5 2 2 +1/2 29. 3 1 0 -1/2 30. 4 3 -2 +1/2 DRAWING PROBLEMS: Four Points Each For problems 31-38: a) Determine the Δ EN and type for each bond. b) Determine the shape of the molecule. c) Draw the molecule and show all charges. d) Determine if the molecule is symmetric or not. e) Determine if the molecule is polar or not. 31. GeBr4 a) 35. PoF2 a) b) b) c) c) d) d) e) e) 32. BiCl3 a) 36. GaHI2 a) b) b) c) c) d) d) e) e) 33. BI3 37. NaH a) a) b) b) c) c) d) d) e) e) 34. CI3Br 38. BF3 a) a) b) b) c) c) d) d) e) e) For problems 39-45: Draw the Lewis Dot Diagrams for each of these COVALENT COMPOUNDS Be sure to include both steps and circle the stick diagram. Four Points Each. 39. CH2CHCHO 42. NSCl 40. SnS2 43. HNO3 (nitric acid) 41. CH3CHNCH3 44. SeO2 45. This one is an Ionic Compound. You only need to show two steps. No stick diagram. CaS 46. This one is an Ionic Compound. You only need to show two steps. No stick diagram. MgF2 47. Determine the hybridization of the second carbon in molecule 39. 48. Determine the hybridization of the central atom in molecule 40. 49. Determine the hybridization of the second carbon in molecule 41. 50. Determine the hybridization of the central atom in molecule 42. 51. Determine the hybridization of the central atom in molecule 43. 52. Count the number of sigma and pi bonds for the molecule in 39. 53. Count the number of sigma and pi bonds for the molecule in 41. 54. Draw all the Resonance structures for SeO3