Ionic Bonding
advertisement

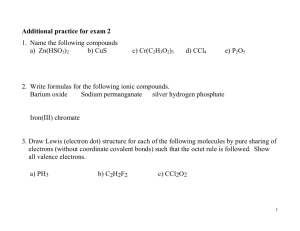
Ionic Bonding Writing Formulae Naming Compounds Atoms and Ions Bond —force that holds 2 atoms together Atoms are neutral=same number of protons as electrons Ion—atom that has lost or gained electrons (it has a charge of + or -) Chemical Remember Only electrons can move Atoms gain or lose electrons to achieve a noble gas configuration To be stable Keeping Track of Electrons The electrons responsible for the chemical properties of atoms are those in the outer energy level. Valence electrons - The s and p electrons in the outer energy level. Core electrons -those in the energy levels below. Keeping Track of Electrons Atoms in the same column have the same outer electron configuration. Have the same valence electrons. Easily found by looking up the group number on the periodic table. Group 2A - Be, Mg, Ca, etc. 2 valence electrons Electron Dot diagrams A way of keeping track of valence electrons. How to write them Write the symbol. Put one dot for each valence electron Don’t pair up until they have to X The Electron Dot diagram for Nitrogen Nitrogen has 5 valence electrons. First we write the symbol. Then add 1 electron at a time to each side. Until they are forced to pair up. N Write the electron dot diagram for Na Mg C O F Ne He Electron Configurations for Cations Metals lose electrons to attain noble gas configuration. They make positive ions. If we look at electron configuration it makes sense. Na 1s22s22p63s1 - 1 valence electron Na+ 1s22s22p6 -noble gas configuration Electron Dots For Cations Metals will have few valence electrons Ca Electron Dots For Cations Metals will have few valence electrons These will come off Ca Electron Dots For Cations Metals will have few valence electrons These will come off Forming positive ions +2 Ca Electron Configurations for Anions * Nonmetals gain electrons to attain noble gas configuration. They make negative ions. If we look at electron configuration it makes sense. S 1s22s22p63s23p4 - 6 valence electrons S-2 1s22s22p63s23p6 -noble gas configuration. Electron Dots For Anions Nonmetals will have many valence electrons. They will gain electrons to fill outer shell. P -3 P Stable Electron Configurations All atoms react to achieve noble gas configuration. Noble gases have 2 s and 6 p electrons. 8 valence electrons . Also called the octet rule. Ar Compounds 2 atoms bonded together Follow the Law of Definite Proportion— have a constant composition—the same # of atoms every time 3 Types of compounds Ionic Bonds Covalent or Molecular Bonds Metallic Bonds Ionic Bonds Made by transferring electrons Made of cations (metals) and anions (nonmetals) The e- lost by the cation is gained by the anion The cations and anions surround each other Smallest piece is a Formula Unit Ionic Bonding Ionic Bonding Anions and cations are held together by opposite charges. Packed into a regular repeating pattern that balances the forces of attraction and repulsion between the ions forming a crystal Very strong bond Ionic compounds are called salts. Properties of Ionic Compounds Crystalline structure. A regular repeating arrangement of ions in the solid. Ions are strongly bonded. Structure is rigid. High melting points- because of strong forces between ions. Crystalline structure Do they Conduct? Conducting electricity is allowing charges to move. In a solid, the ions are locked in place. Ionic solids are insulators. When melted, the ions can move around. Melted ionic compounds conduct. First get them to 800ºC. Dissolved in water they conduct. Ionic solids are brittle + + - + + + + - + + Ionic solids are brittle Strong Repulsion breaks crystal apart. - + - + + - + - + - + Writing the formula for compounds and Naming compounds Learning to speak the language Chemical Formula Shows the kind and number of atoms in the smallest piece of the substance Nicotine C10H14N2 For Ionic Compounds: Systematic Naming There are too many compounds to remember the names of them all Compound-2 or more elements chemically combined Pure substance Name tell how many and what kind of atoms Remember –Ionic Bonds Anions and cations held together by opposite charges Called salts Simplest ratio is called formula unit Bond is formed by transfer of electrons Electrons are transferred to achieve a noble gas configuration Ionic bonding or Formula Unit Shows lowest whole # ratio of atoms in the crystal lattice NaCl MgCl2 Charges on Ions You can tell charge of an atom by its location Elements in the same group have similar properties including the charge when they are ions Oxidation number -the charge of the ion +1 +2 +3 -3 -2 -1 Naming ions We will use the systematic way. Cation- if the charge is always the same (Group A) just write the name of the metal. Transition metals can have more than one type of charge. Indicate the charge with roman numerals in parenthesis. Name these Na+1 Ca+2 Al+3 Fe+3 Fe+2 Pb+2 +1 Li Write Formulas for these Potassium ion Magnesium ion Copper (II) ion Chromium (VI) ion Barium ion Mercury (II) ion Naming Anions Anions are always the same. Change the element ending to – ide F-1 Fluorine Naming Anions Anions are always the same. Change the element ending to – ide F-1 Fluorine Fluor + ide = Fluoride Name these Cl-1 N-3 Br-1 -2 O Ga+3 Write these Sulfide ion iodide ion phosphide ion Strontium ion Polyatomic ions Group of atoms covalently bonded together that gain or lose an electron LEARN YOU POLYATOMIC IONS! Oxyanion –polyatomic ions composed of an element bonded to one or more oxygen atoms – Ate ions – Ite ions – Per – hypo Polyatomic ions Sulfate SO4-2 Sulfite SO3-2 Carbonate CO3-2 Chromate CrO4-2 Dichromate Cr2O7-2 Phosphate PO4-3 Phosphite PO3-3 Ammonium NH4+1 Monatomic vs Polyatomic anions S-2 monatomic Sulfide ion N-3 monatomic Nitride ion Bromide Selenide SO4-2 polyatomic Sulfate ion NO3- polyatomic Nitrate PO4-3 Phosphate ion Naming Binary Ionic Compounds Binary Compounds - 2 elements. Ionic - a cation and an anion. To write the names just name the two ions. Easy with Representative elements. Group A NaCl = Na+ Cl- = sodium chloride MgBr2 = Mg+2 Br- = magnesium bromide Naming Binary Ionic Compounds The problem comes with the transition metals. Need to figure out their charges. The compound must be neutral. same number of + and – charges. Use the anion to determine the charge on the positive ion. Naming Binary Ionic Compounds Write the name of CuO Need the charge of Cu O is -2 copper must be +2 Copper (II) chloride Name CoCl3 Cl is -1 and there are three of them = -3 Co must be +3 Cobalt (III) chloride Naming Binary Ionic Compounds Write the name of Cu2S. Since S is -2, the Cu2 must be +2, so each one is +1. copper (I) sulfide Fe2O3 Each O is -2 3 x -2 = -6 3 Fe must = +6, so each is +2. iron (III) oxide Naming Binary Ionic Compounds Write the names of the following KCl Na3N CrN Sc3P2 PbO PbO2 Na2Se Ternary Ionic Compounds Will have polyatomic ions At least three elements name the ions NaNO3 CaSO4 CuSO3 (NH4)2O Ternary Ionic Compounds LiCN Fe(OH)3 (NH4)2CO3 NiPO4 Writing Formulas The charges have to add up to zero. Get charges on pieces. Cations from name on table. Anions from table (ide) or polyatomic. Balance the charges by using subscripts. Ionic Bonding Na Cl Ionic Bonding + Na Cl - Ionic Bonding All the electrons must be accounted for! Ca P Ionic Bonding Ca P Ionic Bonding +2 Ca P Ionic Bonding +2 Ca Ca P Ionic Bonding +2 Ca Ca P -3 Ionic Bonding +2 Ca P Ca P -3 Ionic Bonding +2 Ca P +2 Ca P -3 Ionic Bonding Ca +2 Ca P +2 Ca P -3 Ionic Bonding Ca +2 Ca P +2 Ca P -3 Ionic Bonding +2 Ca +2 Ca +2 Ca P P -3 -3 Ionic Bonding Ca3P2 Formula Unit Writing Formulas Write the formula for calcium chloride. Calcium is Ca+2 Chloride is Cl-1 Ca+2 Cl-1 would have a +1 charge. Need another Cl-1 Ca+2 Cl2-1 CaCl2 1 (+2) and 2 (-1)=0 Write the formulas for these Lithium sulfide tin (II) oxide tin (IV) oxide Magnesium fluoride Iron (III) phosphide Iron (III) sulfide Writing Ternary Ionic Write the formula for calcium chlorate. Calcium is Ca+2 Chlorate is ClO3-1 Ca+2 ClO3-1 Need another ClO3-1 When you need to have more than one polyatomic ion you must use PARENTHESES Ca(ClO3) 2 Write the Formula Lithium sulfate tin (II) hydroxide tin (IV) cyanide Magnesium dichromate Iron (III) Phosphate Iron (III) sulfide