If energy is released in a rxn, the rxn must be Endothermic
advertisement

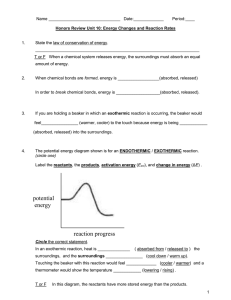
1. If energy is released in a rxn, the rxn must be a. Endothermic c. Catalyzed b. Exothermic d. Balanced 2. A catalyst speeds up a reaction by doing what? a. Increases the energy of the products c. Increases the activation energy d. Decreases the activation energy b. Decreases the energy of the products 3. Draw two graphs. The first one should be an exothermic reaction with a high activation energy. The second should be an endothermic reaction with a low activation energy. 4. Gold has a specific heat larger than aluminum. What which of the following would be true about the same size pieces of gold and aluminum? a. Aluminum would be hotter than gold left outside for 2 minutes b. Gold would be hotter than aluminum left outside for 2 minutes c. They would be the same temperature d. It is impossible to tell 5. A combustion reaction always has what products a. CO2 + a hydrocarbon c. H2O and CO2 b. A hydrocarbon and O2 d. H2O and O2 6. Which of the following is true about an endothermic reaction a. –ΔH c. Releases energy b. Feels cold d. Freezing is an example Use this reaction for questions 7-9: ___Mg + ____O2 ____MgO 7. Which is true about the reaction? a. Is it endothermic c. It has zero activation energy b. It is exothermic d. None of the above are true 8. What type of reaction is this a. Synthesis c. Single replacement b. Decomposition d. Double replacement 9. What would be the correct balancing for the reaction a. 1,1,1 c. 2,1,2 b. 1,2,1 d. 3,2,3 10. What would be the products of this reaction: Li + MgCl2 a. LiCl + Mg c. LiCl + Mg2 b. LiCl2 + Mg d. LiCl2 + Mg2 11. As the pressure of a gas increases, what is true? a. The volume increases c. The number of moles increases b. The temperature decreases d. The temperature increases 12. As the temperature of a gas increases which of the following is true a. The kinetic energy increases c. The volume decreases b. The pressure decreases d. The molecules move slower Use this problem to answer 13-14: How many moles of gas are in a container that has a volume of 2L, a pressure of .45 atm, and a temperature of 10°C. 13. What equation would you use? a. P1V1 = P2V2 c. P1/T1 = P2/T2 b. PV=nRT d. V1/T1 = V2/T2 14. What “R” value would be used? a. 8.3 (kPa*L)/(mol*K) c. 6.4 (mmHg*L)/(mol*K) b. 0.082(atm*L)/(mol*K) d. No “R” value is used 15. What is the temperature in Kelvin? a. -263K c. 283K b. 10K d. 263K