Ideal Gas Law
advertisement

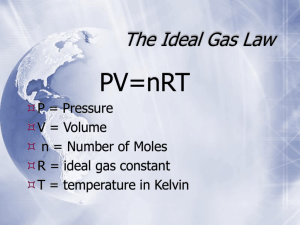
The Ideal Gas Law This gas law combines all four properties of gases - pressure, volume, temperature, and number of moles. The ideal gas law can be used to find any value as long as three of the quantities are known. PV = nRT Conversions K = °C + 273 1 cm3 = 1 mL 1 L = 1000 mL 1 dm3 = 1 L R values: 0.0821 when P is atm 8.31 when P is kPa 62.4 when P is mmHg Standard Conditions 0.00 °C = 273 K 1.00 atm = 760.0 mm Hg = 101.325 kPa = 101,325 Pa 1. How many moles of gas are contained in a typical human breath that takes in 0.50 L of air at 1.0 atm pressure and 37oC? Answer: 0.020 mol 2. If a person exhales 25.0 g of CO2 in an hour, what volume does this amount occupy at 1.00 atm and 37oC? Answer: 14.5 L 3. How many moles of gas are contained in a compressed air tank for scuba diving that has a volume of 7.0 L and a pressure of 210 atm at 25 oC? Answer: 60 mol 4. Assuming a fixed amount of gas complete the following table. a. b. c. P 19.5 atm 500.0 kPa 0.0477 atm V ?L 250. mL 15,200 L n 4.7 x 104 mol 0.120 mol ? mol T2 300. oC ? oC -15 oC 5. Incandescent light bulbs "burn out" because their tungsten filament evaporates, weakening the thin wire until it breaks. Argon gas is added inside the bulbs to reduce the rate of evaporation. What is the pressure in atmospheres of 3.4 x 10-3 moles of Ar in a 75 mL light bulb at 20 oC? Answer: 1.1 atm Ideal Gas Law: Molar Mass and Density Using an expanded form of the ideal gas law, the density and molar mass may be calculated. d = PM RT M = gRT PV M = dRT P 1. What is the density of argon gas at 80.8 oC and 131 kPa that is in the incandescent light bulb? Answer: 1.78 g/L 2. Aluminum chloride sublimes at high temperatures. What density will the vapor have at 225 oC and 0.939 atm pressure? Answer: 3.06 g/L 3. Automobile airbag inflates when NaN3 is converted to Na and N2. a. Write a balanced equation for the reaction that occurs. b. What type of reaction does this represent? c. What volume of nitrogen gas would be produced if 100. g of NaN 3 reacts at 0.975 atm and 30.0 oC Answer: 4. A gas, 4.0 g, occupies 11.2 L at 2 atm and 273 K. What is the molar mass of the gas? What is the identity of the gas? Answer: 4.00g/mol, He 5. Krypton gas does a better job than argon of slowing the evaporation of the tungsten filament in an incandescent light bulb. Because of its higher cost, however, Kr is only used when longer life is considered to be worth the extra expense. a. How many moles of Kr must be added to a 175 mL light bulb to yield a gas pressure of 117 kPa at 21.6 oC? Ans.: 0.00836 mol b. What is the volume of the light bulb that contains 1.196 g Kr at a pressure of 1.70 atm and 97 oC. Ans: 0.255 L c. What is the density of the Kr gas at 18.2 oC and 762 mm Hg? 3.51 g/L